Pfizer/BioNtech And Moderna MRNA Covid-19 Vaccines Closely Mimic The Immune Response Of Natural SARS-CoV-2 Infections
(Posted on Wednesday, February 24, 2021)
NEW YORK, NEW YORK – FEBRUARY 22: Anya Harris prepares a Moderna coronavirus (COVID-19) vaccine at Red Hook Neighborhood Senior Center in the Red Hood neighborhood of the Brooklyn borough on February 22, 2021 in New York City. Deaths from coronavirus
GETTY IMAGES
As more and more variants of SARS-CoV-2 either emerge or make landfall in the United States, the question of whether they’ll be able to bypass the immune defenses we’ve acquired against Covid-19 becomes increasingly pressing.
The official record states that nearly 30 million people across the United States have had Covid-19 at some point in the past year—resulting in, at least in theory, a certain degree of natural immunity—while about 44 million and counting have received at least one dose of a vaccine. Though the immunology of Covid-19 is complex and only partially understood, a recent study comparing the characteristics of naturally occurring and mRNA-induced antibodies against the new variants offers some much-needed insight into the difficulties we can expect to encounter in our attempts to protect ourselves going forward.
The study, published in Nature earlier this month, examines the immune responses of 20 participants vaccinated against Covid-19, comparing them to people who were naturally infected. Of the 20, 14 received the Moderna vaccine and 6 received the Pfizer-BioNTech vaccine. Researchers measured levels of both antibodies and memory B cells, as well as their duration over time.
Antibodies and memory B cells
Two months after volunteers received the second of two injections required for full immunization, the researchers found that their IgG antibodies persisted longer than their IgM and IgA counterparts, typical of most immune responses to both infection and vaccination. They also found that the IgG and IgM levels noted in vaccinees surpassed those of the naturally infected patients, whose samples were obtained 1.3 months after initial infection, then a second time five months later, consistent with previous reports.
The researchers also measured memory B cells, which like IgG antibodies play a critical role in sustaining immunity. Almost immediately following immunization, the antibody response naturally decreases, with the half life of antibodies lasting anywhere from six weeks to three months. Within a year, protection from disease is guaranteed only by memory cells. In this study, the amount of memory B cells specific to the receptor-binding domain recorded in convalescents 1.3 months following infection was higher than that of vaccinees, but about the same after six months. Other studies have found, however, that memory B cells from natural infection increase over time.
Variants vs. vaccines
Though further confirmation that the Moderna and Pfizer-BioNTech vaccines generate a robust immune response is always welcome, when new variants are thrown into the mix the results are less promising. Researchers investigated this particular piece of the puzzle by pitting antibody-loaded plasma from the vaccinees against lab-generated SARS-CoV-2 spike proteins containing some of the defining mutations of emergent variants, including N501Y, K417N, and E484K (Figure 1). All three of these mutations appear either singularly or simultaneously in the B.1.1.7 (UK), B.1.351 (South Africa), and P.1 (Brazil) variants, and all three are either suspected or known to play a role in increasing chances of immune evasion and person-to-person transmission.
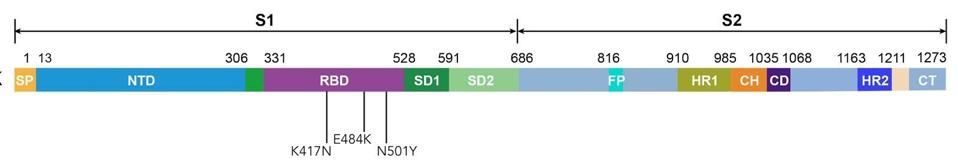
Figure 1. Linear representation of the spike protein with mutations N501Y, K417N, and E484K.
AUTHOROnce again, the results of accinees and convalescents from natural infection proved similar—though not as encouraging. According to the paper, vaccinee sera about three times less effective against viruses carrying a combination N501Y, K417N, and E484K, while convalescent sera fared even worse. For convalescent sera obtained about a month after infection, the average decrease in potency was 2.9. For six-month-old sera, it was 4.3.
The antibodies’ lackluster performance against mutated spike proteins is a cause for concern. Though memory B cells can usually be depended upon to produce protective antibodies against proteins recognized earlier, the response takes several days to be effective. Memory responses do not typically prevent reinfection for the original or variant strains. The memory response often protects those reinfected from serious disease. The question of whether or not the immune memory will be sufficient to protect against disease upon reinfection by a variant strain is an open question.
Variants are reported to cause serious disease upon reinfection of those previously infected. The similarity of the natural and vaccine immune response is cause for concern, not reassurance in this circumstance. Likewise vaccines that raise effective immune responses to the original spike protein are not always effective protecting against infection by a variant strain. These observations raise profound questions regarding Covid-19 control strategies.
Variants vs. monoclonal antibodies
In another portion of the study, the researchers selected the most potent of the monoclonal antibodies, sorted them into provisional classes based on neutralizing activity, and tested them individually or in combination against the SARS-CoV-2 variants. The vast majority of the antibodies were less effective against viruses containing the E484K mutation by a factor of at least tenfold—a finding that echoes those of previous research, in particular another preprint study led by Washington-based researchers that came out in early January. An equivalent reduction in efficacy was seen in some of the antibodies matched against Kr17N and N501Y mutations as well.
The researchers examined the nature of mutations that allowed the spike protein to evade neutralization by a set of monoclonal antibodies. They found that three of antibodies selected for mutations at the 501 site (including N501Y), six selected for mutations at the 417 site (including K417N), and five selected for E484K specifically. These observations recall the theory of parallel evolution that I’ve discussed in previous writings—the notion that the same mutations, facing comparable selection pressures, can arise independently but concurrently in individual patients, in vitro laboratory experiments, and global populations.
Summary
What this study confirms is that antibodies, whether generated by vaccines or by primary infection or reinfection, fade over time and cannot be expected to protect us from primary infection. We’ll have to rely on the strength and speed of immune memory, which in itself contains a large number of unknowns, including whether or not it will be effective against variants that have evolved to evade the secondary immune response.
The implications all point to one broader conclusion: that current and future variants of SARS-CoV-2 demand a certain level of adaptability from the drugs and vaccines we’re relying on to prevent Covid-19. If we want to save ourselves from another catastrophic winter, we need to be able to beat the virus at its own game—or, at the very least, keep pace with its maneuvers, just as we do with influenza. Otherwise, we risk leaving ourselves vulnerable once again, despite knowing full well what we’re in for.
Originally published on Forbes (February 24, 2021)

