If I Had Covid-19, Should I Still Get Vaccinated? Absolutely
(Posted on Thursday, February 25, 2021)
Doctor Marie Msika Razon prepares doses of the AstraZeneca Covid-19 vaccine before vaccinating patients aged over 50 and suffering from a comorbidity, at her medical office in Paris, on February 25, 2021. – Private practitioners in France started
AFP VIA GETTY IMAGES
The number of people who have received at least one dose of a Covid-19 vaccine is growing steadily greater by the day. While most of those vaccinated thus far belong to high-priority, high-risk groups—older adults, health workers, educators, and so on—in due time a much broader swathe of the population will gain eligibility, including the many millions of us who have caught and recovered from the disease at least once since the pandemic began.
Though at least some level of natural immunity likely persists among those who had Covid-19 before, the emergence of SARS-CoV-2 variants that are not only more transmissible but probably also more challenging for the immune system to overcome has put a damper on mass vaccination efforts that depend almost entirely on technologies created using the original Wuhan strain. Just as there exists a large possibility that vaccines will have to be modified to keep pace with viral variation, previously infected individuals may be more susceptible to reinfection by the new variants—bad news for everyone, certainly, but especially for those who had a long or life-threatening battle with the disease the first time around.
At least one preprint study can provide some clarification on the subject—not to mention some encouraging findings. According to the study, which was uploaded to the preprint server medRxiv in early February, the neutralizing antibody response of individuals who had Covid-19 previously strengthened considerably—by 1000-fold, to be precise—following their first injection of a Pfizer or Moderna mRNA vaccine. Their antibodies not only neutralized both the Wuhan and South Africa (B.1.351) strains, but in some cases SARS-CoV-1 as well.
To understand the wider implications of these findings, we must unpack them piece by piece. The study enlisted ten previously infected men and women in the Seattle area, the majority in their 40’s or 50’s. Seven received a shot of the Pfizer-BioNTech vaccine, while the remaining three received the Moderna vaccine. On average, about 200 days had lapsed between the day the participants first reported Covid-19 symptoms and their first clinical pre-vaccine visit. Pre-vaccination data was collected a couple weeks before their first shot, followed by post-vaccination data around two weeks after.
In terms of their laboratory studies, the researchers began with some baseline antibody neutralization experiments that compared the effectiveness of monoclonal antibodies against the original Wuhan spike protein, on the one hand, and a South Africa (B.1.351) spike on the other, which included the increasingly prevalent and problematic mutations E484K, N501Y, and K417N. The mutated spike was more resistant to neutralization than the original, with the potency of the monoclonal antibodies pitted against it decreasing as much as tenfold. This finding is consistent with previous reports, though it bears mentioning that mutations occurring in other parts of the virus, such as the N-terminal domain and accessory genes, weren’t mentioned in the research paper and may have been left out of this study.
The next step for the researchers was to test whether blood plasma collected from the study’s participants pre- and post-vaccination would fare just as poorly against the South Africa variant. They focused on the binding responses of IgG, IgM, and IgA antibodies specific to the receptor-binding domain, as well as any changes in antibody concentration. (Binding, it must be noted, is a mechanism related to but separate from neutralization, which will be discussed shortly.) The convalescent sera—that is, the pre-vaccination antibody response—responded variably and rather unpredictably to both the original and mutated strains, the immune response of one donor so slight as to be undetectable. Following immunization, antibody binding signals increased 50-fold across the board, but in the case of the South Africa spike they were much lower.
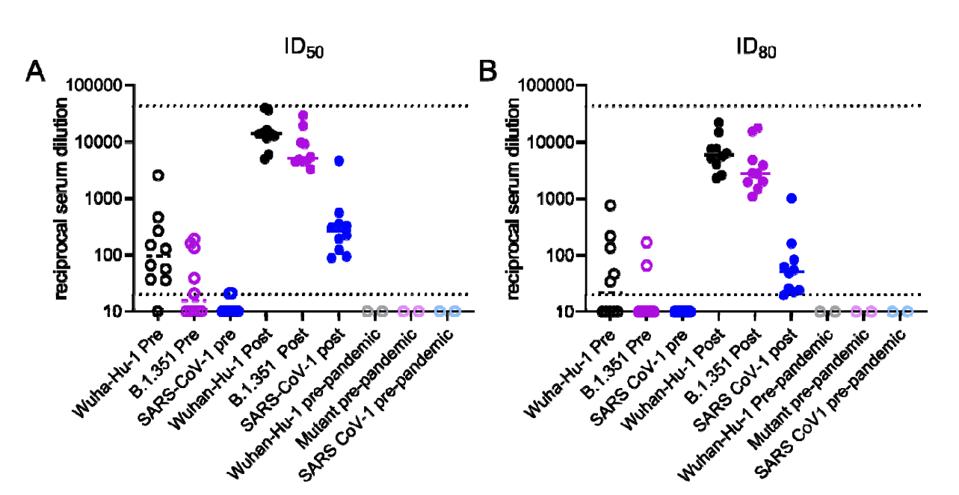
Figure 1. Neutralizing activities in serum from recovered COVID-19 donors prior to and following a single immunization with the Pfizer/BioNTech or Moderna vaccines against Wuhan-Hu-1 and B.1351 SARS CoV-2 pseudoviruses and SARS-CoV-1 pseudoviruses.
“ANTIBODIES ELICITED BY SARS-COV-2 INFECTION AND BOOSTED BY VACCINATION NEUTRALIZE AN EMERGING VARIANT AND SARS-COV-1” HTTPS://WWW.MEDRXIV.ORG/CONTENT/10.1101/2021.02.05.21251182V1The difference in binding strength pre- and post-vaccination, as well as from one spike to the next, was large enough to beg the question of whether measurements of neutralization would proceed in a similar fashion. While pre-vaccination samples of convalescent sera from nine of the 10 participants were able to neutralize the Wuhan spike, only five could sufficiently neutralize the South Africa spike. Fewer still—two out of those five—reached 80 percent neutralization or higher. But in the weeks following vaccination, concentrations of neutralizing antibodies against both strains leapt 1000-fold. They were even effective against lab-generated SARS-CoV-1.
Thanks to this study, three different levels of immunity against Covid-19 now have precedent—natural immunity, vaccine-mediated immunity, and a combination of both facilitated by a single vaccine dose. The last of these three appears to be most potent by a wide margin. Though further investigation is needed to know whether that potency is sustained over a period of several months, the fact that it only took half the standard two-dose vaccination regimen to elicit such a large jump in neutralizing capability is extremely promising. If corroborated by more data, this finding could change the way we allocate vaccines.
The study also has potential implications for drugmakers, particularly those developing monoclonal antibody drugs. I recommend them to take a closer look at the antibodies that were cross-reactive against not just the Wuhan and South Africa strains, but SARS-CoV-1, too. More than anything, what will allow us to continue protecting ourselves against Covid-19 in the face of dangerous new variants is our ability to use studies like this one to make smarter and more informed decisions, both in our individual lives and our national policies.
Originally published on Forbes (February 25, 2021)

