New Antibody Therapy And Prophylactic Shows Promise In Defending Against SARS-CoV-2 Variants Of Concern
(Posted on Monday, April 26, 2021)
As SARS-CoV-2 variants grow in type and frequency, Covid-19 researchers are on the hunt for parts of the virus that remain consistent across variants in order to create Covid-19 treatments that work for multiple strains of the virus. This is the first in a series discussing these potential Achilles’ heels for Covid-19.
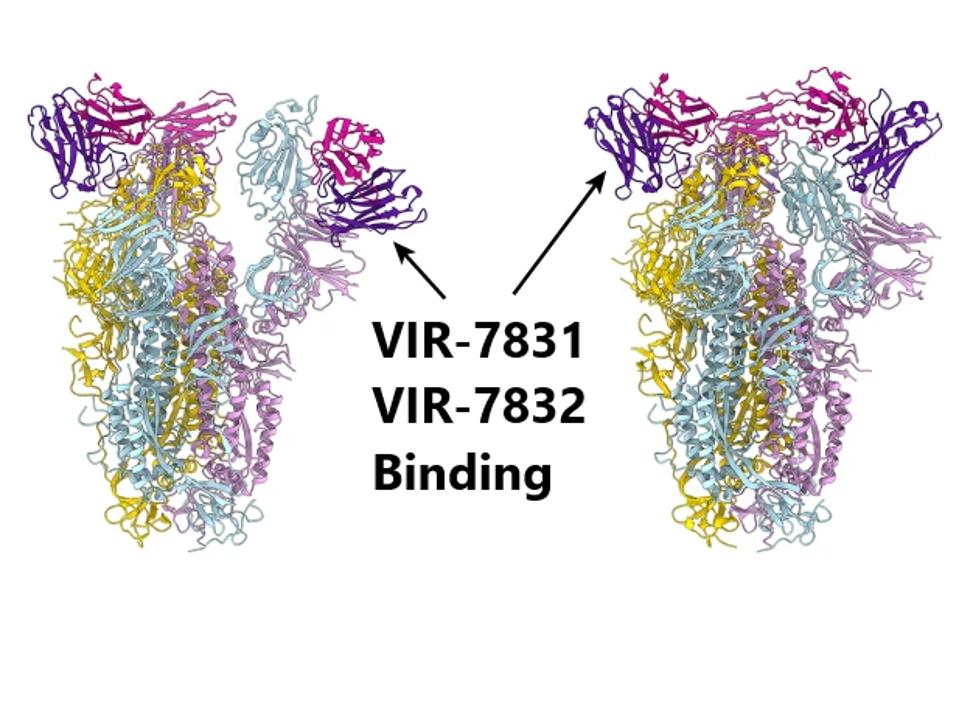
Sites for VIR-7831 and VIR-7832 binding
PINTO ET AL // NATURE
In a search for an Achilles’ Heel for the virus, the scientific world is monitoring potential targets for virus neutralization. One of these targets is a highly conserved region of the receptor-binding domain in the spike protein and two new monoclonal antibodies targeting this region are rapidly advancing in development and trial. The first of Vir Biotechnology and GSK’s two experimental antibodies, VIR 7831, aims to be used as a monotherapy for the early treatment of Covid-19 in adults at high risk of hospitalization. Early trial returns showed an 85% reduction in hospitalization or death, indicating the potential success of the antibody. The second, VIR 7832, is still in the preliminary stages of study as a dual-action prophylactic that potentially blocks viral entry to healthy cells and enhances clearance of infected cells. These antibodies are highly engineered and merit deeper analyses, as they could have wide-ranging therapeutic and prophylactic success against Covid-19, including SARS-CoV-2 variants, in the near future.
Work began on these antibodies as early as April and May 2020 as researchers searched for neutralizing monoclonal antibodies for SARS-CoV that may carry over effectiveness to SARS-CoV-2. One SARS-CoV antibody extracted from B-cells in a SARS-CoV patient, S309, potently neutralized both SARS-CoV and SARS-CoV-2, prompting a deeper study of the antibody and the epitope to which it binds.
The S309 antibody recognizes a proteoglycan epitope on the receptor-binding domain of SARS-CoV-2. The antibody is composed of 6 complementarity-determining regions (CDR) loops which come in contact with amino acids 337-344, 356-361, and 440-444 in the spike protein. Two of these CDRs, CDRH3 and CDRL2 sandwich the glycan of the SARS-CoV-2 spike protein at position N343, making it a significant point of contact for the antibody.
The reason S309 carries over its neutralizing effects from SARS-CoV is the significant conservation of contact amino acids between viruses. Of the 22 residues involved in antibody binding, 17 are strictly conserved, four are conservatively conserved, ad one is semi-conserved between viruses. This research concludes by noting the potential of S309 as a Covid-19 countermeasure and that variants of S309 that may have boosted half-lives and effector functions have already entered accelerated development.
Early in 2021, GSK and Vir Biotechnology began work on the S309 antibody to optimize it for SARS-CoV-2 prevention and treatment, resulting in VIR-7831 and VIR-7832. Both antibodies were mutated to contain an “LS” mutation in the Fc region to prolong antibody half-life and potentially enhance distribution to the respiratory mucosa. This would block viral entry to greater effect and would provide protection against infection for longer.
The VIR-7832 antibody additionally contains an Fc GAALIE mutation shown to boost T-cell immunity. GAALIE is an acronym for the amino acid mutations in the Fc region, specifically G236A, A330L, and I332E. These mutations would enable antibody-dependent complement-mediated cytotoxicity without inducing antibody-dependent enhancement. In other words, it would neutralize the virus and clear out infected cells faster.
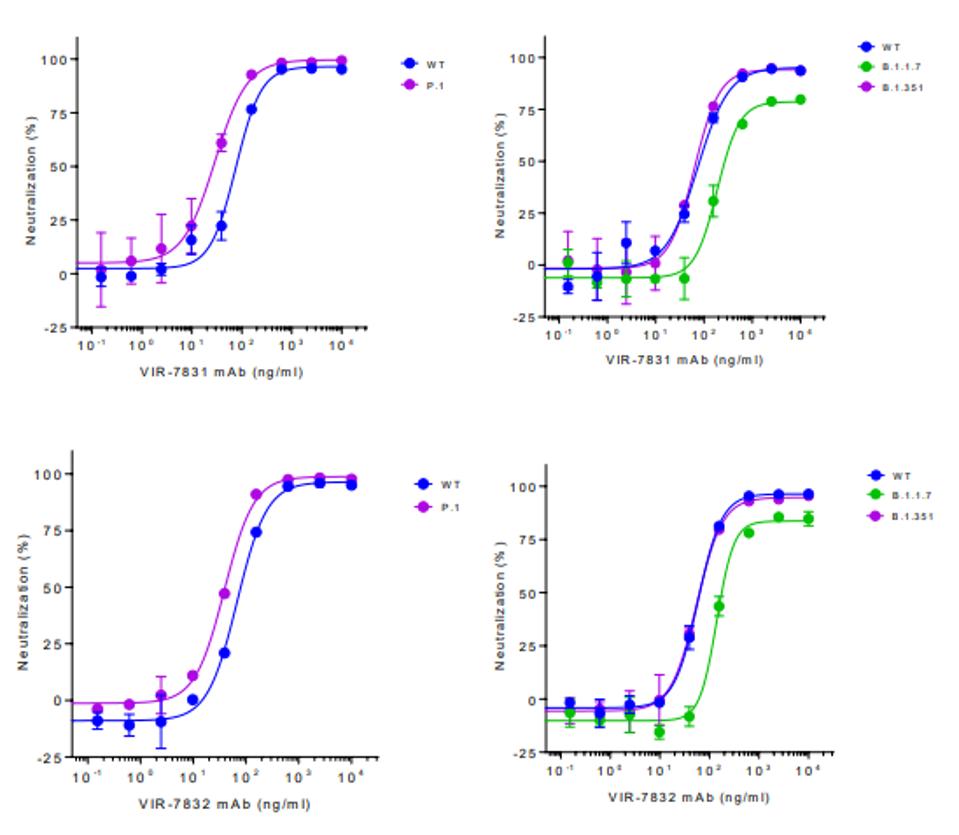
Early trials indicate that these antibodies effectively neutralized wild-type SARS-CoV-2 at highly effective levels. Additionally, and perhaps more notably, both antibodies additionally neutralize the United Kingdom B.1.1.7, South Africa B.1.351, and Brazilian B.1.1.28.1 variants at high levels. The Brazilian and South African variants are neutralized as well as the wild-type and the United Kingdom variant neutralized about 25% less effectively for both VIR-7831 and VIR-7832, as displayed in the figure below.
As a word of caution, there are now variants with mutations in the highly conserved target region that these antibodies attack. While B.1.1.7, B.1.351, and B.1.1.28.1 are all mostly conserved in the regions 337-344, 356-361, and 440-444, there are a number of sequenced viruses in the GISAID database which carry mutations at some of these locations. The most frequent of these are at position 440 where there are 1,689 mutants, position 357 where there are 699 mutants, and position 344 where there are 307 mutants. While these numbers are relatively low in comparison to the 1.2 million sequences in GISAID and the millions of infections worldwide, India has taught us that a strong and resistant virus can spread rapidly. Some of these mutations may have a large effect on antibody resistance, for instance, some mutations to P337 and E340 reduce the neutralizing capability of the antibodies up to 200-fold, showing that this potential Achilles’ heel may have an Achilles’ heel of its own.
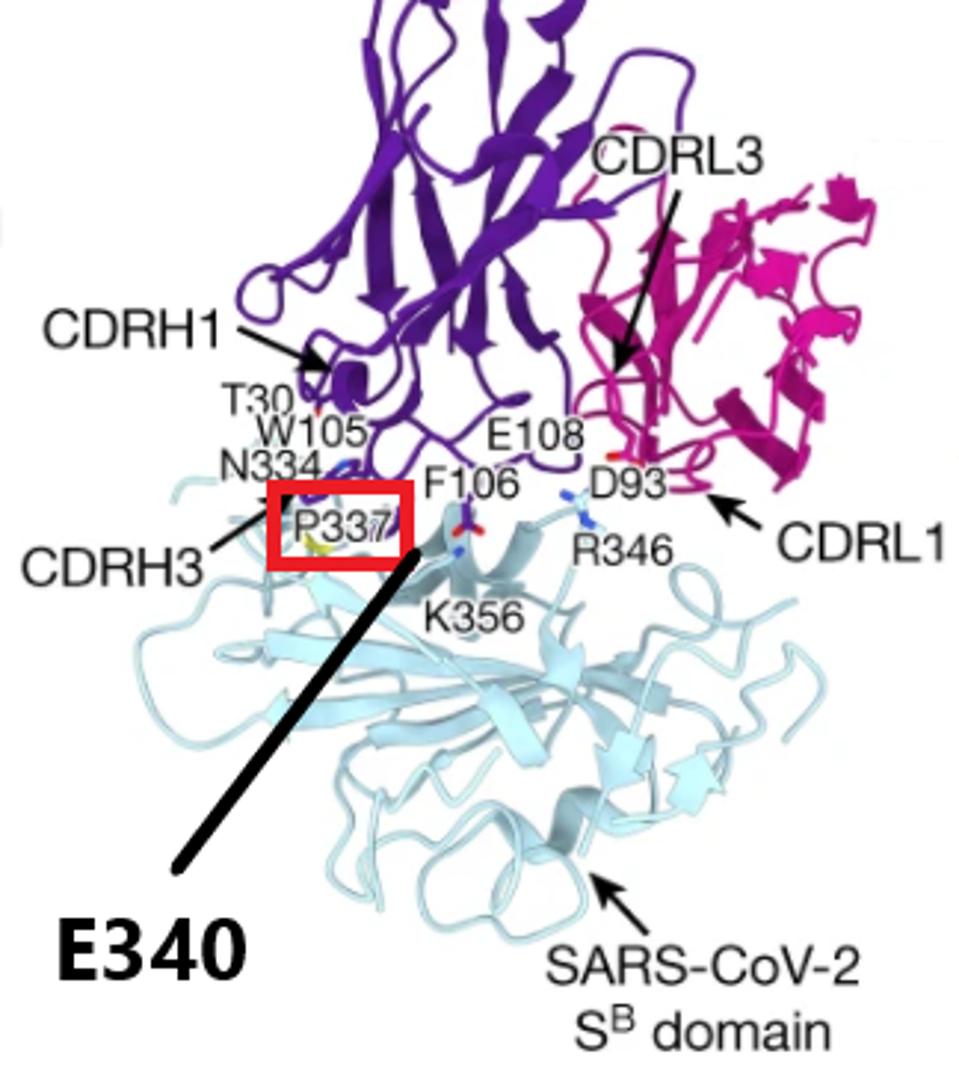
Antibody binding to the virus with major mutation sites highlighted
PINTO ET AL // NATUREThese antibodies are ultimately a promising step towards furthering Covid-19 therapeutics ad prophylactics. They show strong neutralization of the wild-type virus and some of the major variants of concern. However, there are mutants out there that may be resistant to VIR-7831 and VIR-7832. We suggest more research on these antibodies could clear up concerns about mutations in the conserved region. While this targeted region is not the ultimate Achilles’ heel, it may turn out to be a good starting point as we explore more possibilities in future additions to this series.
Read the full article on Forbes.
Originally published on April 26, 2021.

