The Growing Threat Of The Delta Pluses At Home And Abroad
(Posted on Thursday, October 28, 2021)
This is the third in a series examining the past, present, and future of the pandemic and viral variants.

Massive dark storm clouds dump rain over the wide plains and dirt tracks of the Maasai Mara National Reserve in Kenya.
GETTY
Each successive variant of the SARS-CoV-2 virus to date was more transmissible than the last. New variants drove multiple waves of Covid-19 throughout the world. Here, examine what happened in the past and present of the pandemic in order to understand what may lie in our future.
First, there was the Triad variant that drove infections in the Summer of 2020. Then, there were regional variants of interest and concern such as Alpha, Beta, and Gamma that drove infections in the Winter of 2020 into 2021. Most recently, Delta is the major variant fueling infections around the world.
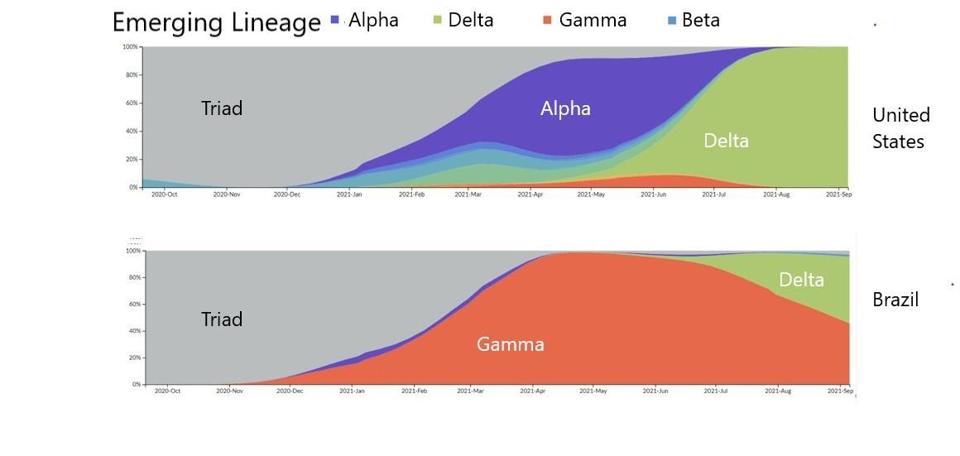
FIGURE 1: Distribution of emerging lineages in SARS-CoV-2 infections in the United States and Brazil.
STEBBING
Delta seems to have similar properties to the Triad variant, in that it is now being displaced by different regional variants that appear to be more transmissible. For example, akin to how the more transmissible Delta variant displaced the Alpha variant, an even more transmissible variant of Delta—AY.4—displaced the original Delta. More recently, a new variant—AY.4.2—appears to be displacing both, as shown in Figure 2. In the United States, Delta seems to be regionally displaced by a number of AY variants, one of which—AY.33.1—appears more transmissible than its predecessors.
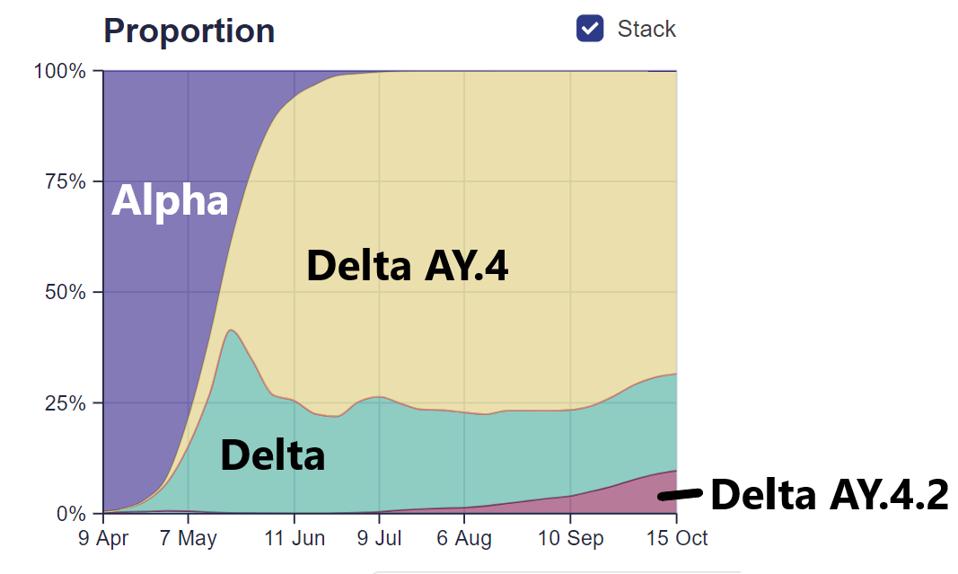
FIGURE 2: Proportion of SARS-CoV-2 infections by different viral variants sequenced in England from early April to mid-October.
SANGER INSTITUTE
The ongoing theme throughout the pandemic is that variants are selected for increased transmission. Some variants vary by antibody neutralization capability, whether by natural infection or vaccine. The South African-originated Beta variant is one example. The primary driver, however, is clearly transmissibility.
What we mean by transmissibility is how readily the virus transmits from one person to another. This is influenced by a number of factors, including but not limited to the stability of the virus in the air, the avidity of the virus Spike protein to the host cell, the efficiency and concentration to which the virus replicates, and how days a person is contagious. By all accounts, the Delta variant excels in all categories, replicating faster and producing greater concentrations in nasal fluids. Delta is neutralized about as well as predecessor strains, so its universal dominance for the past several months lies squarely with its transmissibility.
The question that now arises is whether there are currently or will be variants of Delta that transmit even more rapidly, akin to the Gamma variant described in part two. Recent reports suggest the answer is a qualified yes. According to the GISAID SARS-CoV-2 sequence database, Delta has more than 40 sequenced sublineages.
The AY.4 Delta Plus Variants
Some Delta sublineages are labeled ‘AY.#’. Many thousands of individual Delta variants are cataloged in an international repository named GISAID. One of the Delta sublineages, AY.4, has outpaced the original B.1.617.2 strain (Delta) in terms of directed infectious strains. The AY.4 sublineage has caused more infections worldwide than the original Delta variant. Following the virological pattern of Gamma, Delta has developed a number of Delta Plus variants that are likely more transmissible and potentially more immune evasive than the original that came before.
Here we describe two Delta sublineages of note: AY.4/AY.4.2 fueling new cases in Europe and AY.33 that appears to be spreading more rapidly than others the American Northeast
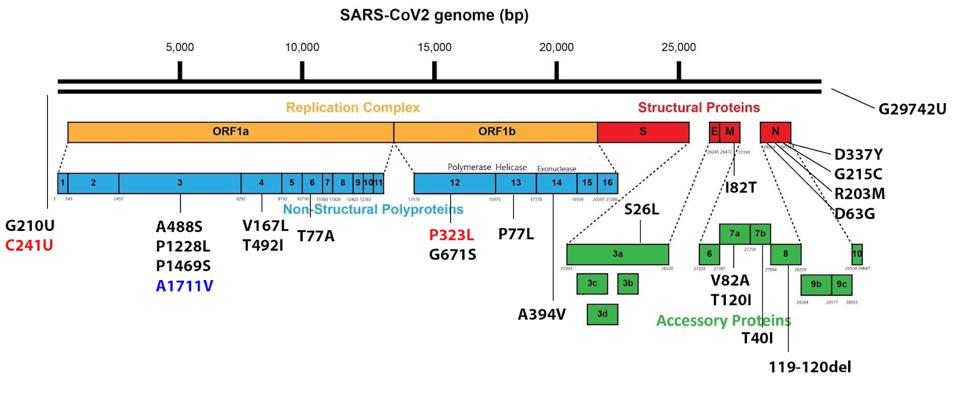
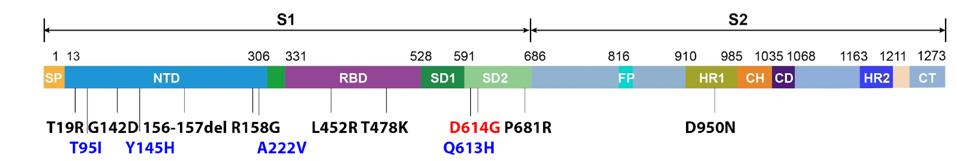
FIGURE 3: Original Delta genome and Spike protein mutations. Mutations in red are canonical to the Triad variant; Mutations in black are canonical to the original Delta variant; Mutations in blue are Delta plus mutations in AY.4, AY.4.2, and AY.33.1.
ACCESS HEALTH INTERNATIONAL
The figure above illustrates the mutations found in the original Delta variant that emerged in India earlier this year. It differs significantly from the Triad variant that fueled infections in the Summer of 2020. Denoted as B.1.617.2, the variant contained 27 amino acid mutations and four amino acid deletions. While a detailed study of these individual mutations is, as of yet, unavailable, we speculate that each may play some role in increases in transmissibility, immune evasion, replication, and pathogenesis.
With each continuous passage of the virus through a new host, new mutations are selected for their impacts in these listed viral traits. If the mutations are successful, they are maintained and new variants are formed. This is how the Gamma variant developed into a number of “Gamma Plus” sublineages and is how Delta is now divided into dozens of Delta Plus sublineages.
The previously mentioned AY.4 variant, for instance, contains all the original Delta mutations shown in Figure 1, but contains an additional Spike protein mutation—T95I—and an additional mutation in the replicative complex proteins—NSP3 A1711V. This variant rapidly overtook the original Delta strain to fuel cases over the past several months in Europe and elsewhere. The mutation of only two amino acids yielded a virus that was significantly more transmissible than the original Delta variant, reaching similar levels of infection in less time.
While Delta swiftly overtook Alpha as the dominant strain, AY.4 with its two additional mutations rapidly predominated in a matter of weeks. The simple amino acid substitutions appear to yield a significantly more transmissible variant.
In the bottom right of the figure, there is a growing presence of another variant. Just as AY.4 altered two amino acids to improve its viral efficiency, a new variant, AY.4.2, seems to have done the same. Using the AY.4 template, this new Delta Plus variant adds two additional Spike mutations to its genomic makeup—Y145H and A222V. Early data indicates that the variant is around 10-15% more infectious than previous major strains. Following suit with the AY.4 Spike mutation, these lie in the N-terminal domain. This follows a pattern of continued modification in this region, as also discussed with the Gamma Plus sublineages. Nearly every major variant of concern or interest to this point of the pandemic has contained at least one, and often multiple N-terminal domain mutations. We note that major changes to the charge and polarity in this region could have significant effects on virus affinity to target cells, as well as the efficiency monoclonal and convalescent antibodies can bind to the virus.
If our past experience with variants is prologue, the AY.4.2 Delta Plus variant may drive yet another wave of infections. The AY.4 Delta sublineage overtook Delta rapidly, as did the Gamma Plus sublineages. There is some evidence that suggests we may be witnessing a similar phenomenon.
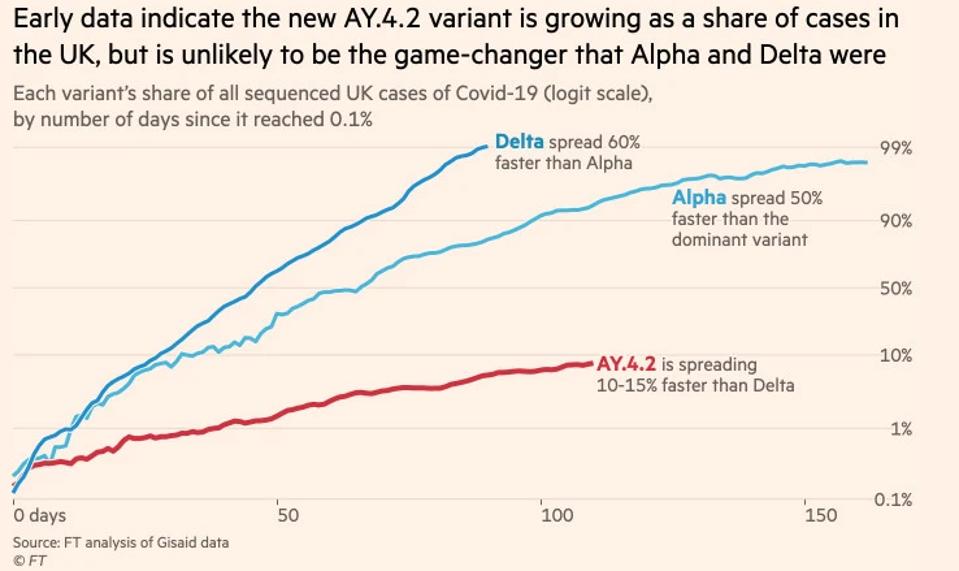
FIGURE 4: AY.4.2 as a share of cases in the United Kingdom as compared to the Alpha and original Delta variants.
FINANCIAL TIMES
Reports have emerged noting AY.4.2 is now representative of about 6% of infections in the United Kingdom. The frequency of infection of AY.4.2 doubles every 28 days. Moreover, the AY.4.2 is now present in dozens of countries, including the United States, Russia, and Israel. Current waves of infection in Eastern Europe, such as the Baltics, Germany, Hungary, may be driven by this variant.
The AY.33.1 Variant
In addition to AY.4.2, there is a new variant growing in the Northeastern United States that is beginning to look like a cause for concern. AY.33.1 is growing in infection frequency in the Northeast and is another independent derivative of the Delta variant. Just like AY.4 and AY.4.2, AY.33.1 maintains the mutational framework of B.1.617.2 and thus the transmissibility advantages it already displays.
The key mutation of note in AY.33.1 is Q613H within the Spike protein. The mutation is located immediately before the Triad mutation D614G. This close proximity suggests that Q613H is a key mutation that may impact infectivity, particularly by the stabilization of the S1/S2 complex after cleavage and the favoring of an open, rather than closed, configuration for the receptor-binding domain—the configuration required for infection. Early indications from the Northeast suggest that AY.33.1 has between a 10 to 20% replication advantage over Delta itself.
While AY.4.2 and AY.33.1 have yet to infect the sheer volume of people Delta or Alpha had in similar timeframes, likely due to widespread vaccinations, it may still come to infect in greater numbers. Recent studies suggest that antibody titers from mRNA vaccination wane heavily after six months. Vaccinations peaked in April and May, meaning the six-month deadline is arriving for many people in the immediate future. While third-shot vaccinations are promising, many may not be in a rush to get another dose due to speculation that it is necessary or whatever other reason. This could aid Delta sublineages with a winter wave as the weather grows cooler and populations move to indoor spaces.
In conclusion, we urge that government agencies and the scientific community continue and expand their efforts to understand virus variation globally. Expanded sequence surveillance programs could give us an early warning, allowing us to prepare in advance for the next devastating wave of infections.

