Hope For Those Aching Joints
(Posted on Friday, October 29, 2021)
This story is part 3 of an occasional series on the current progression in Regenerative Medicine. In 1999, I defined regenerative medicine as the collection of interventions that restore to normal function tissues and organs that have been damaged by disease, injured by trauma, or worn by time. I include a full spectrum of chemical, gene, and protein-based medicines, cell-based therapies, and biomechanical interventions that achieve that goal.
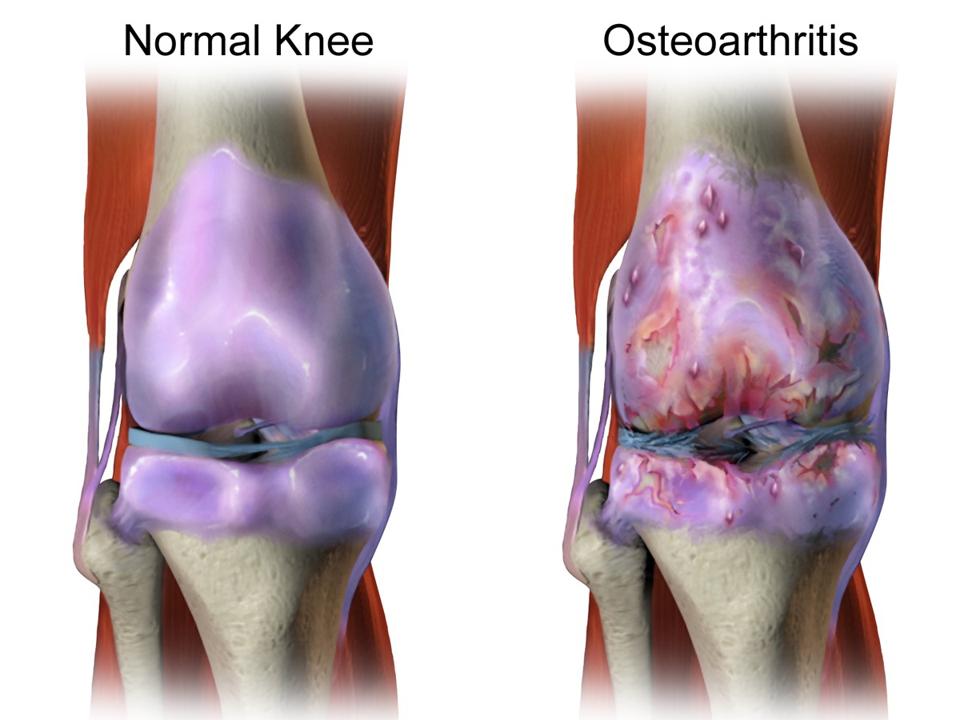
Knee with healthy cartilage beside knee with deteriorated cartilage.
WIKIMEDIA COMMONS
At long last, there seems to be real hope in rebuilding damaged articular cartilage. Researchers from the University of Southampton recently discovered a new method to generate cartilage tissue from stem cells. Articular cartilage covers the ends of bones and acts as a shock absorber in the joints. It provides a smooth, low friction surface and allows for painless movement. However, it is known to be particularly susceptible to degradation through sports injuries or general wear and tear. Actions such as falling, moving a joint in a strange direction, or even wearing high heels for extended periods of time can all contribute to the deterioration of cartilage and increased pain in movement.
Past studies have been conducted to grow cartilage tissues without scaffolds but they were unable to grow tissues that were larger than 1 mm in size. Scaffolds are support frames that tissues are grown onto. They promote greater growth of tissues that lack structural integrity, such as cartilage, but can cause complications when implanted into patients. It is crucial to develop methods to grow larger amounts of structurally sound cartilage tissue without scaffolds.
Griffith et al. began their experimental procedure with human embryonic stem cells. Human embryonic stem cells are in their early stages of cell development. When nudged in the right direction, these stem cells can be transformed into practically any cell in the human body. The researchers were particularly interested in promoting the growth of a cell type called chondrocytes.
Chondrocytes are the most abundant cell type present in cartilage and are known to grow more efficiently in environments with low oxygen levels. Researchers differentiated the stem cells within a low oxygen environment. They also introduced supplemental growth factors throughout the generation process of the tissue to further promote the growth of cells.
These modifications resulted in significant improvement in the efficiency of chondrocyte growth and ultimately cartilage tissue formation. The lab-grown cartilage tissue exhibited the same biomechanical properties as human cartilage and displayed key signals for the production of articular cartilage—SOX9 and Type 2 collagen. SOX9 is a gene required for chondrocyte cell production. Type 2 collagen is the primary protein present in articular cartilage. Unfortunately, much like previous studies there was a limit to the growth of the tissue. After 19 weeks, the cartilage tissue grew only slightly greater than 1 mm in diameter and approximately 3 mm in area.

Stem cell-derived cartilage growth at 4 weeks and 19 weeks.
NATURE, L.A. GRIFFITH, K. M. ARNOLD, B.G. SENGERS, ET AL.
To address the barrier of structural integrity, researchers attempted to co-culture the cartilage tissue on human articular cartilage. The human cartilage used was derived from osteoarthritis patients after hip replacement surgery. The human cartilage tissue was trimmed to 1 cm sections. After growing stem cell-derived cartilage tissue for 4 weeks in a lab, these lab-grown tissues were placed on the human cartilage and co-cultured for an additional 16 weeks.
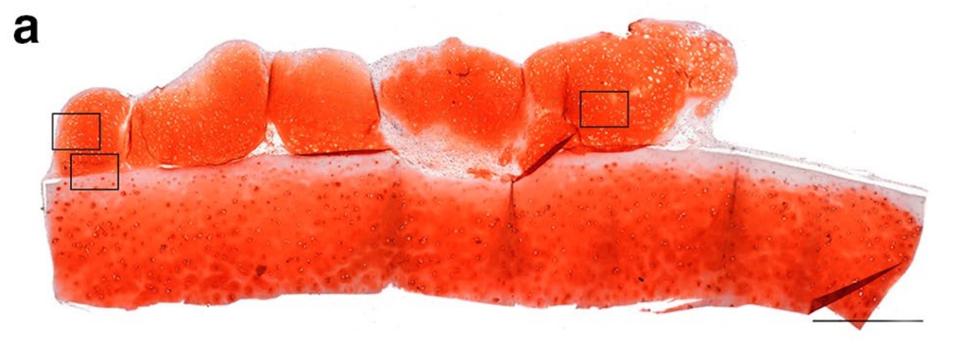
Human articular cartilage co-culture.
NATURE, L.A. GRIFFITH, K. M. ARNOLD, B.G. SENGERS, ET AL.
The resulting tissue grew substantially, reaching six times the size of initial experiments. In addition, the tissue continued to display the crucial SOX9 and Type II Collagen proteins characteristic of human articular cartilage.
After determining their ability to increase the size of lab-grown cartilage tissues, researchers were interested in developing a method of growth that did not require human articular cartilage samples. They decided to test the growth of their cartilage tissues on a particular type of membrane (polyethylene terephthalate-transwell membrane) that very closely resembles the surface of human articular cartilage. After culturing the 4-week-old stem cell-derived tissue on the membrane for 16 weeks, the resulting tissue was 4.5 mm in diameter and matched the characteristics seen in human articular cartilage.
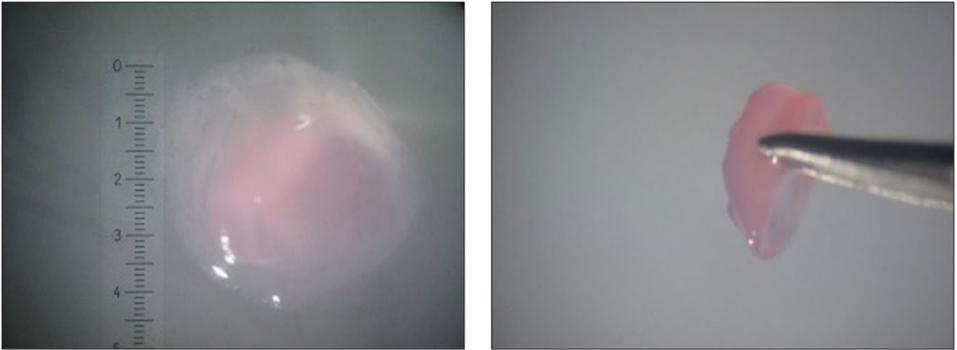
Polyethylene terephthalate-transwell membrane grown cartilage.
NATURE, L.A. GRIFFITH, K. M. ARNOLD, B.G. SENGERS, ET AL.
The successes of the human tissue co-culture and the transwell membrane culture beg the question: what qualities of human articular cartilage or transwell membranes allow the stem cell-derived tissues to grow so large? The next steps in this study will be to continue investigating why these results occurred so researchers can further develop methods to grow cartilage that is optimized for a human scale.
This study is a significant step forward for research involving the recovery of damaged cartilage. With this progress, researchers have provided a concrete foundation for the development of new treatments that have the potential to successfully treat injuries, aid in healthy aging, and improve lives all over the world.

