Spike And Nucleocapsid Protein Mutations In Tanzanian And Ugandan Strains Of SARS-CoV-2 Demand Attention
(Posted on Friday, November 12, 2021)
Tanzania and Uganda in East Africa
GTREVIEW.COM
Virus variation is a major driver of current infections. All SARS-CoV-2 variants sweeping almost all areas of the world today, including variants of concern Alpha, Beta, Gamma, and Delta, originally derived from the Triad variant (D614G). This variant was the first major variant of the Wuhan strain and includes D614G in the Spike (S) protein, P323L in the NSP12 polymerase, and C241U in the 5’ untranslated region.
We have previously noted two outliers that differentiate from the Triad template, both originating in East Africa. These are the A.23.1 strain in Uganda and the A.30 strain from Tanzania. These two variants warrant detailed analysis as they could provide insight into the range of variations that could yield new waves of the Covid-19 pandemic. In that regard, we analyze a new paper by Arora et al. that compares the ability of one of these strains, A.30, which contains a highly mutated S protein, to avoid neutralization by antibodies derived from convalescent sera, monoclonal antibody treatment, or vaccine.
A.30, which was first detected in patients arriving at an Angolan airport from Tanzania, contains 34 amino acid changing mutations throughout its various viral proteins. In the S protein alone, there are ten amino acid substitutions and five deletions.

FIGURE 2: Spike mutations regularly found in the A.30 genome.
ARORA ET AL.
For the purposes of antibody neutralization, the researchers focus on the mutations which affect two major domains of the S protein, the N-terminal domain and the receptor-binding domain. The researchers note that all A.30 deletions along with four substitutions are found in the N-terminal domain of the surface unit S1. The residue positions of these deletions and substitutions lie in an antigenic supersite that is targeted by most neutralizing antibodies.
Arora et al. investigate antibody-evasive features of A.30 by creating rhabdovirus pseudotypes bearing the SARS-CoV-2 S protein. These viral models can be used to measure antibody neutralization in vitro, reducing the risk of experimenting with live viruses.
Cellular Host Range of A.30
To test the immune resistance of these mutations, Arora et al. first implanted the A.30 S mutations on rhabdoviral pseudotype viruses. They then exposed the pseudotypes to five different targets to model different organs in a human host. To compare, pseudotypes of the Beta and Eta S proteins are also created and exposed to human-like cell targets. Notably, A.30 seemed to infect some cell types more effectively than others, namely Vero (kidney), 293T (kidney), Huh-7 (liver), and A549 (lung). The authors conclude that A.30 exhibits a cell line preference not observed for other viral variants, possibly changing the disease profile of those infected.
Neutralization by Monoclonal Antibodies
The infected cells are then treated with bamlanivimab and etesevimab, both separately and in conjunction via antibody cocktail, to emulate Covid-19 therapy. Consistent with previous experiments, they found that Beta was resistant to both bamlanivimab and etesevimab, whereas Eta was resistant to only bamlanivimab. A.30, along with Eta, was bamlanivimab resistant, but susceptible to etesevimab and a bamlanivimab + etesevimab cocktail.
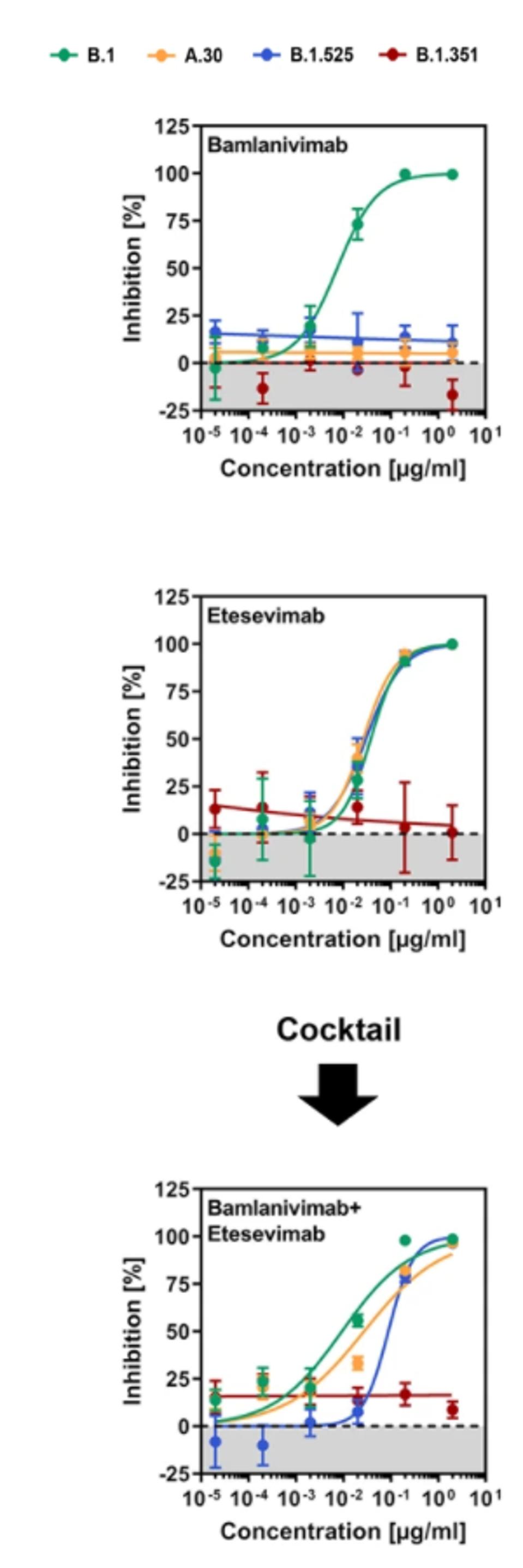
FIGURE 3: Neutralization of SARS-CoV-2 B.1, A.30, B.1.525, and B.1.351 by monoclonal antibodies used for COVID-19 therapy.
ARORA ET AL.
Neutralization by Convalescent Sera
Next, the researchers compared neutralization of the virus by convalescent sera developed through previous infection. Their findings suggest that the Beta variant was the most resistant to convalescent sera, followed by A.30, and then Eta, though all three African variants are more resistant than the Triad variant.
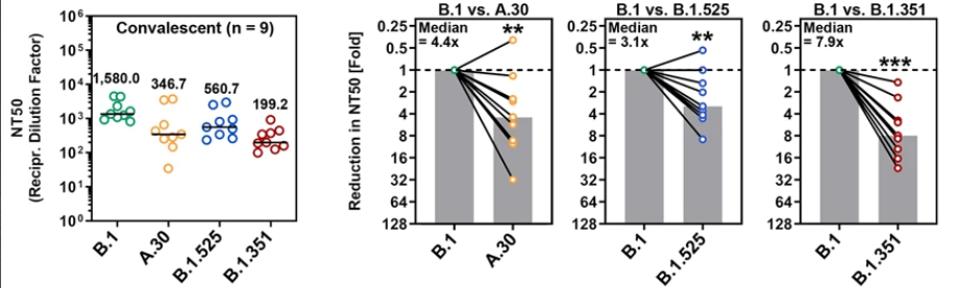
FIGURE 4: Neutralization of SARS-CoV-2 B.1, A.30, B.1.525, and B.1.351 by antibodies in convalescent plasma.
ARORA ET AL.
Neutralization by Vaccine-administered Antibodies
Finally, the researchers analyzed the neutralization of each variant by antibodies administered by vaccination, both by adenovirus vaccines like AstraZeneca and mRNA vaccines like Pfizer. This data is the most interesting in reference to the A.30 variant. Arora et al. note that A.30 was significantly more resistant to neutralization by homologous ChAdOx1 nCoV-19 (Astrazeneca) or BNT162b2 (Pfizer) vaccination antisera than either Beta or Eta, by roughly half an order of magnitude.

FIGURE 5: Neutralization of SARS-CoV-2 B.1, A.30, B.1.525, and B.1.351 by antibodies in the AstraZeneca and Pfizer vaccines.
ARORA ET AL.
The researchers conclude by noting that the potential spread of the A.30 variant warrants close monitoring and rapid installment of countermeasures. We echo this sentiment.
We note that other factors besides S protein mutations may be involved in the A.30 variant’s immune resistance. While there are 15 mutations in the A.30 S protein, there are 19 mutations in non-S proteins throughout the A.30 genome.

FIGURE 6: A.30 mutations found outside the Spike protein.
ACCESS HEALTH INTERNATIONAL
N Protein Mutations
A recent manuscript demonstrates that single point mutations in the specific region of the Nucleocapsid (N) protein, position 199-205, substantially increase the infectivity of the virus, in some cases by over 150-fold. The A.30 variant contains a mutation in this region, S202N, which was not specifically studied in the aforementioned paper, but is remarkably similar to another, S202R, which conferred an increase of infectivity roughly 50-fold. Notably, both A.30 and A.23.1 appear to contain the S202N mutation independently.

