A New Monoclonal Antibody That Has The Potential To Neutralize All Viral Variants
(Posted on Wednesday, November 24, 2021)
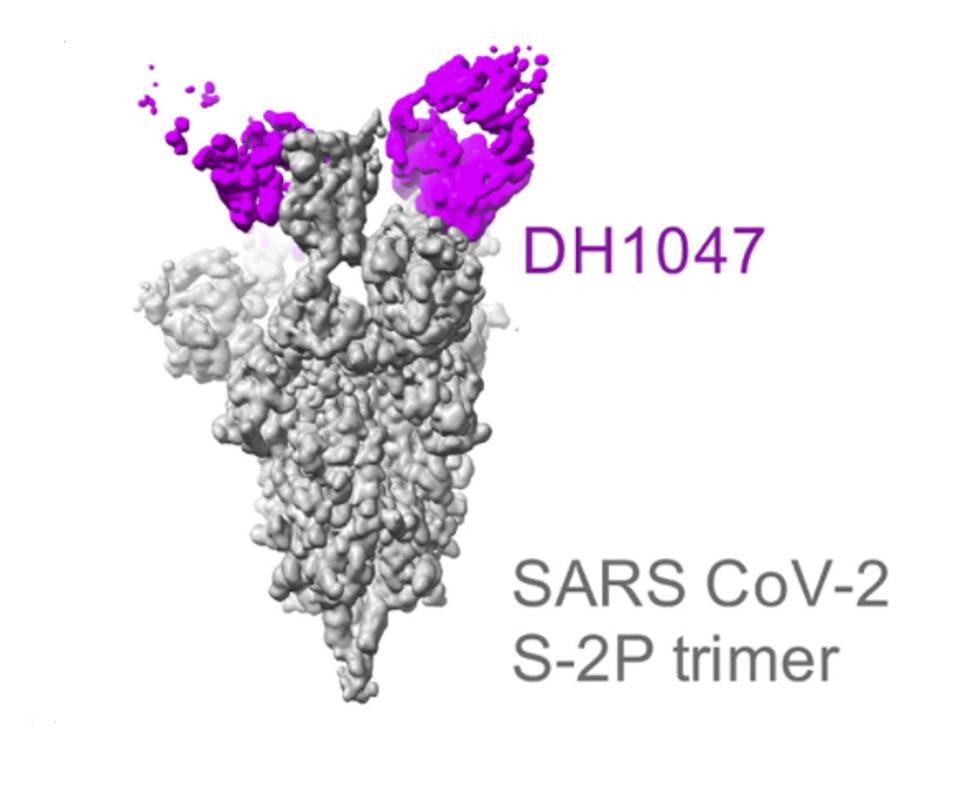
Cryo-electron microscopy of neutralizing and non-neutralizing Abs in complex with SARS-CoV-2 Spike ectodomain structures of SARS-CoV-2 S protein in complex with DH1047
DAPENG ET AL.
Monoclonal antibodies offer what is now a proven way to prevent and treat Covid-19 infections. Until recently, antibodies were administered by intravenous (IV) injection, which limited their widespread use. Newer technologies allow antibodies to be administered intramuscularly or subcutaneously. Additionally, new data indicates that with minor modifications, monoclonal antibodies can provide protection for many months. These advances add to the ability to treat and prevent Covid-19.
One limitation of Covid-19 monoclonal antibodies is that virus variants can escape neutralization. Single point mutations or deletions in certain areas of the SARS-CoV-2 genome can eliminate or gravely reduce antibody potency. Here we describe a novel antibody: DH1047. The distinguishing characteristic of this antibody is that it is capable of neutralizing all known variants of interest and concern by targeting highly conserved epitopes.
Martinez et al. at the University of North Carolina aimed to identify, isolate, and analyze an RBD-targeting monoclonal antibody with the potential to neutralize beyond just the SARS-CoV-2 wild type. Building on the research by Rappazzo et al. that noted that an engineered RBD-directed antibody, ADG-2, could neutralize and protect against multiple SARS-related viruses, Martinez et al. hypothesized that conserved RBD glycans could create potent targets for cross-reactive antibodies.
Identifying DH1047
Thousands of antibodies develop within us upon infection with an invading pathogen. To uncover their winning antibody, Martinez et al. built on the foundations of previous research. Beginning with a pool of 1737 monoclonal antibodies isolated from SARS-CoV and SARS-CoV-2 convalescent sera described by Li et al., Martinez et al. then focused on 50 which bound to both SARS-CoV, SARS-CoV-2, and other animal sarbecoviruses such as bat virus WIV-1.
To further restrict their candidates, Martinez et al. tested the 50 antibodies for neutralizing activity on a mouse-adapted SARS-CoV-2 virus, a SARS-CoV-1 virus, bat coronavirus WIV-1, and bat coronavirus RsSHC014. These tests resulted in four primary candidates that displayed broadly neutralizing capability: DH1235, DH1073, DH1046, and DH1047.
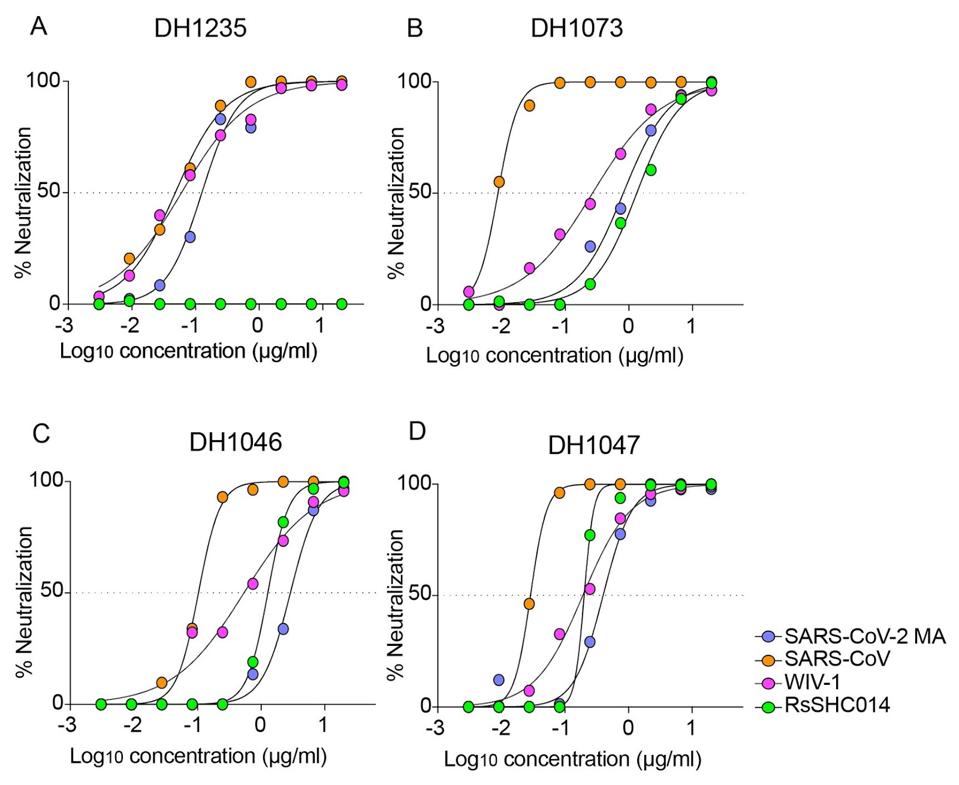
FIGURE 1: The genetic relationships of ACE2 and non-ACE2 using sarbecovirus receptor binding domains is shown. SARS-CoV-2 2AA MA is shown in purple, SARS-CoV is shown in orange, WIV-1 is shown in pink, and RsSHC014 is shown in green. The scale bar
MARTINEZ ET AL.
Martinez et al. further analyzed their refined group of four against several more sarbecoviruses, including horseshoe bat viruses RaTG13-CoV and RsSHC014, pangolin virus GXP4L-CoV, camel virus MERS-CoV, human cold virus HuCoV OC43, and others. Notably, the four antibodies bound to some viruses but not others. Specifically, they only bound to Group 2B betacoronaviruses, leaving out viruses like MERS-CoV. Martinez et al. conclude that this is likely due to the targeted epitope of the four antibodies existing in Group 2B betacoronaviruses, but not others.
Martinez et al. then aimed to analyze the protective activities of the four antibodies to determine whether their binding and neutralization carried over to animal models. Neither DH1235, DH1073, nor DH1046 protected infected mice from SARS-CoV lung viral replication, demonstrating a contrast to the potent neutralization displayed in vitro. DH1047, however, fully protected mice subjects from lung virus replication. Further tests indicated that DH1047 as a prophylactic prevented illness-related weight loss and pulmonary complications as well. Martinez et al. had found their winner.
DH1047 Binding
Using cryo-electron microscopy, Martinez et al. observed the techniques DH1047 and its related antibody use to bind to a broad range of sarbecoviruses so effectively. They note three antibody fragments bound to three SARS-CoV RBDs in the “up” position. This is the position required for virus interaction with the host cell. The residues making antibody contact are 356-372, 390-404, and 488-492. Two of these sets, 356-372 and 390-404, are located in the receptor-binding core, whereas 488-492 is located in the receptor-binding motif, which is the part of the receptor-binding domain that makes contact with the host ACE2 receptor. Martinez et al. further note that these sets of residues closely resemble that of the SARS-CoV-2 spike and others, defining the cross-vulnerability of sarbecoviruses.

FIGURE 2: Linear representation of the SARS-CoV-2 receptor-binding domain with DH1047 binding regions noted in blue.
ACCESS HEALTH INTERNATIONAL

FIGURE 3: Cryo-electron representation of DH1047 binding to the SARS-CoV/SARS-CoV-2 Spike protein.
MARTINEZ ET AL.
DH1047 SARS-CoV-2 Variant Neutralization
Perhaps the most immediate use for a broadly neutralizing cross-sarbecovirus antibody is the neutralization of pervasive mutated SARS-CoV-2 variants. Using pseudovirus neutralization assays, Martinez et al. introduced DH1047 to several variants, including the Triad (or D614G), Alpha, Beta, Gamma, B.1.429, B.1.526, Kappa, and Delta. They found that in the case of all SARS-CoV-2 variants, the 50% neutralization assay values ranged between 0.1214 and 0.1609 micrograms per milliliter. In other words, DH1047 potently neutralized all SARS-CoV-2 variant pseudoviruses. They also tested the antibody against live virus samples of the Triad, Alpha, and Beta variants. Again, 50% neutralization assay values were low, running from 0.059 to 0.111 micrograms per milliliter, confirming that DH1047 broadly neutralizes SARS-CoV-2 variants.
Conclusion
We look forward to the rapid development of this antibody and others like it. Cross-neutralizing antibodies such as DH1047 would make an important contribution to the control of the Covid-19 pandemic.

