The Many Faces Of Omicron
(Posted on Wednesday, July 6, 2022)
This is part of a continuing series describing antiviral antibodies to prevent and treat SARS-CoV-2 infections. In this series, we will discuss the fundamental nature of virus evolution, how SARS-CoV-2 has mutated to evade neutralizing antibodies, and our latest attempts to fight against these mutations with more recent and improved antibody candidates.
The latest versions of Omicron leading the charge of recent new cases are BA.4, BA.5, and BA.2.75. Since its discovery in late 2021, dozens of variants of the original Omicron strain have caused hundreds of millions of infections. Every strain carries a slightly different genetic sequence due to mutations developed over time. These mutations are sometimes inconsequential but often impact major viral characteristics including infectivity and immune evasion.
Omicron BA.1 contains 30 mutations in the Spike protein alone. Later versions of Omicron expand on this vast array. These mutations were a significant factor in Omicron’s rapid rise this past winter, infecting roughly one million people in the United States daily at its peak. These mutations increase the transmission of the virus in the population, whether vaccinated or not, by more than tenfold as compared to the original Wuhan strain.
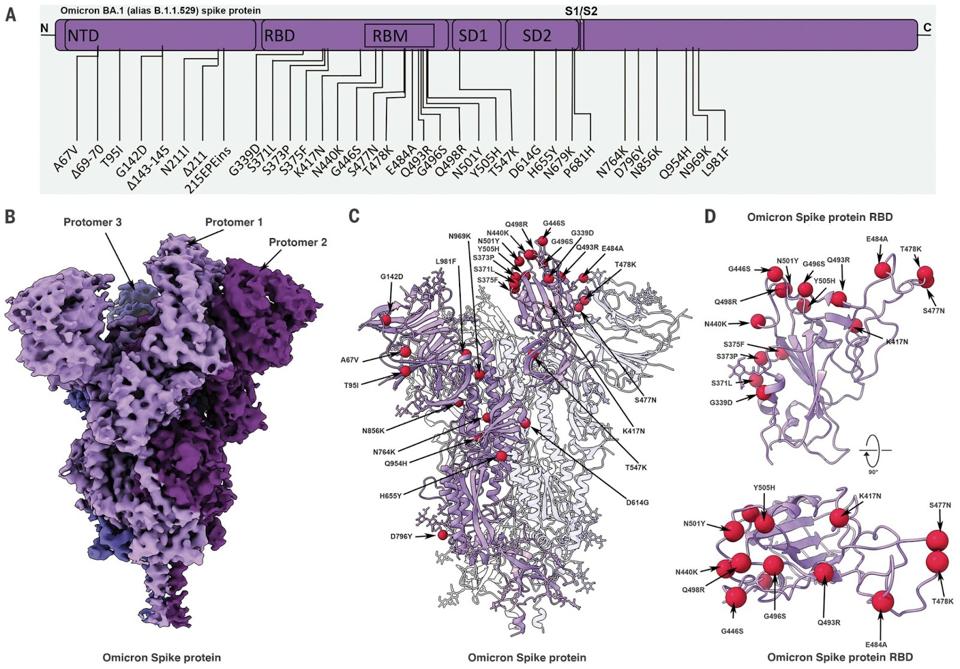
FIGURE 1: Cryo-EM structure of the Omicron BA.1 spike protein. (A) A schematic diagram illustrating the domain arrangement of the spike protein. Mutations present in the Omicron variant spike protein are labeled. (B) Cryo-EM map of the Omicron spike
MANNAR ET AL.
It is worth noting that Omicron has a number of mutations outside the Spike protein as well. These impact viral characteristics including replication efficiency, pathogenesis, and virulence. There are 19 mutations in proteins other than the Spike throughout the genome, including Orf1a, Orf1b, E, M, Orf8, and N.

FIGURE 2: BA.1 Non-Spike mutations.
ACCESS HEALTH INTERNATIONAL
On top of its infectivity is Omicron’s immune evasion of natural immunity, vaccine-induced immunity, and monoclonal antibody immunity, notably for this discussion. Monoclonal antibodies target specific structures, often in the Spike protein. For the first year of the pandemic, scientists made antibodies for the original Wuhan version of SARS-CoV-2. However, with every Spike mutation, the structure of Spike slightly changes.
Think of a key in a lock. They made a key for the lock, but the lock changed shape in the meantime. Then the scientists created a new key for the new lock, but the lock was constantly shifting, and the cat-and-mouse game continued.
An early study on the neutralization of Omicron BA.1 by various approved and in-progress monoclonal antibodies found that 26 of 29 lost some or all neutralizing potency against the new strain. Later Omicron subvariants likely have increased immune escape and may reduce the potency of these antibodies even further.
Neutralizing efficiency only seems to be growing worse as Omicron evolves into more mutated strains such as the latest BA.4 and BA.5. These later variants are more heavily mutated in the receptor-binding domain than BA.1, meaning antibodies that target the receptor-binding domain will have a tougher time binding and neutralizing the new variants.
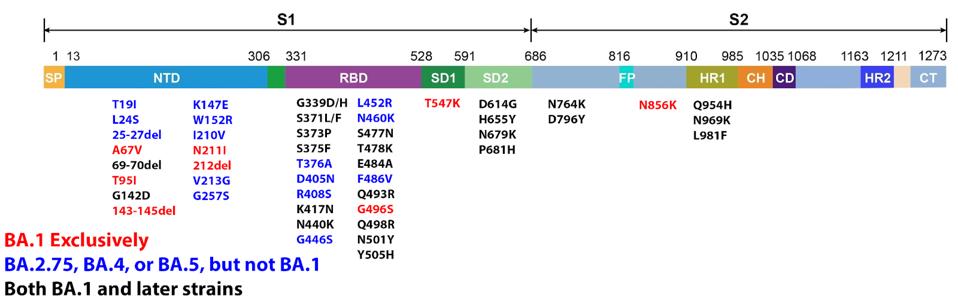
FIGURE 3: Omicron BA.1, BA.4, BA.5, and BA.2.75 Spike proteins compared.
ACCESS HEALTH INTERNATIONAL
The continued mutation of Omicron is also leading to lower vaccine potency against later strains. For instance, three doses of Pfizer vaccination is almost 3x less effective against BA.4 or BA.5 than it is against BA.1, BA.2, or BA.3.
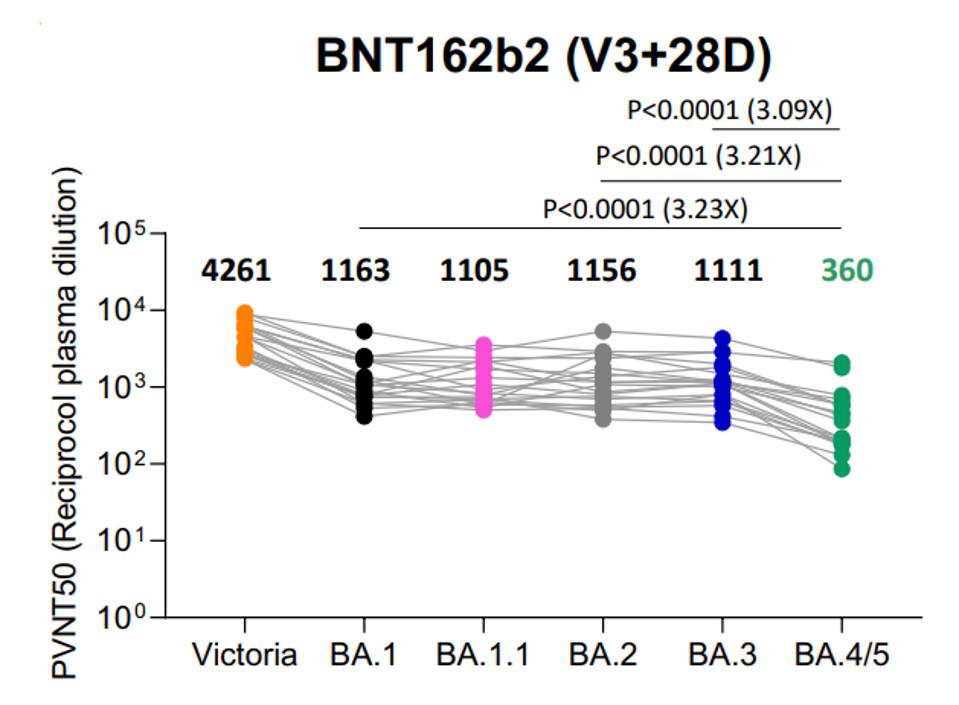
FIGURE 4: IC50 values for the indicated viruses using serum obtained from vaccinees 4 weeks after the third dose of Pfizer BNT162b2.
TUEKPRAKHON ET AL.
All is not lost. Among those that can neutralize Omicron was 35B5, a potent, broadly-neutralizing monoclonal antibody effective against all known variants. Rather than making a new key for the lock, 35B5 essentially blows the lock off the door. This antibody targets specific amino acids in the N-terminal domain of the Spike that are regularly unmutated. This indicates that the unmutated structure at those positions is critical for virus function, such as N165 and N234, which act together as a molecular switch for the Spike’s changing up and down conformations.
By targeting unmutated positions in not only Omicron but all variants, 35B5 breaks the lock off the door, future-proofing against more heavily mutated variants that may come our way in the coming months or years. In our opinion, all monoclonal antibodies should pursue the broad-neutralization strategy. Three more recently unveiled antibodies have shown promise in early in vitro testing.
The first two, Cv2.1169 and Cv2.3194, were isolated by researchers at the Pasteur Institute in recent months. Both antibodies are notable because they cross neutralize both BA.1 and BA.2, indicating a wide net of neutralization. Cv2.1169, specifically, demonstrated therapeutic efficacy in animal models as well.
The third, SP1-77, was recently detailed by a team of researchers at the Harvard Medical School and Duke University Medical School. This antibody also cross-neutralizes BA.1 and BA.2, as well as all other variants of concern. The unique antibody was generated via humanized mouse model and blocks membrane fusion rather than RBD-binding. The more weapons in our arsenal, the better.
We will also describe the latest in the Omicron family: BA.2.75. You may find descriptions for other notable Omicron family members, such as BA.4, BA.5, and BA.2.12.1 in previous articles.

