CAR T Therapy to Treat and Cure Rheumatoid Arthritis
(Posted on Saturday, December 24, 2022)
Here we describe the use of CAR T therapy to treat mice with experimental rheumatoid arthritis. Previous installments discuss the fundamentals of CAR T and its applications for B cell cancers, multiple myeloma, lupus and the heart, as well as the use of antibody switches to control CAR T cell activation and new research on combination CRISPR/CAR T therapy.
Digital xrays of both hands showing severe rheumatoid arthritis affecting both wrists and hands.
GETTY
A study published in the Journal of Immunology advances work towards a cure for rheumatoid arthritis via cell mediated therapies. Around 1.3 million people in the US have this autoimmune disease. The condition can severely impair quality and can reduce life expectancy by 3 to 10 years. The study builds upon a recent cancer innovation to effectively target the cells associated with disease progression. Although the results have not yet been verified in humans, the findings demonstrate the burgeoning potential of this treatment to address autoimmunity.
Autoimmunity and Rheumatoid Arthritis
Rheumatoid arthritis (RA) differs from the “wear and tear” arthritis most recognize. Rheumatoid arthritis occurs when the immune system wrongly attacks the body’s own joint tissues and leaves widespread inflammation in its wake. Figure 1 clearly illustrates how the injured joint tissue swells and erodes the bone. The affected joints often become painful, stiff and red as a result. Further side effects include fever, fatigue and weight loss.
Existing treatment options usually involve medicines to suppress the immune system and slow down disease progression. However, these treatments do not address the underlying cause: the errant and self-reactive immune cells.
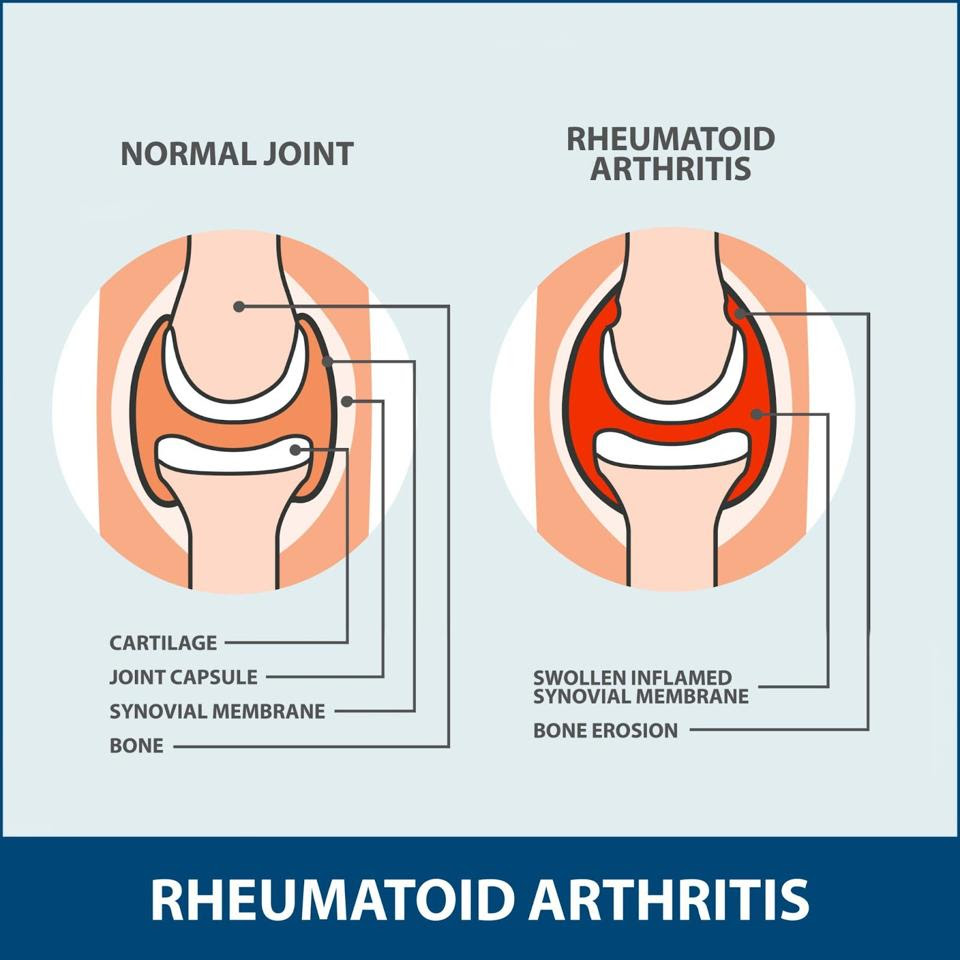
FIGURE 1: With rheumatoid arthritis, the immune system attacks the synovial membrane, the thin tissue encapsulating the joint; the membrane becomes inflamed and causes bone erosion.
FLORIDA ORTHOPEDIC INSTITUTE
Haywire Immune Mechanism
Current understandings of autoimmune arthritis suggest that the disease relates to a set of genes called human leukocyte antigen (HLA) class II. Many versions of this gene exist. In general, reading these genes produces a variety of molecules which can present biological tags to nearby helper T cells, also known as CD4+ T cells.
The system works similarly to a flag and pole. The biological tag—formally known as an antigen (Ag)—acts as a flag; the flag cannot be read unless a major histocompatibility (MHC) class II molecule lifts it up for helper T cells to see. Corresponding helper T cells can then recognize their “flag,” bind to it, and trigger an immune response.
Considering that particular helper T cells react and progress rheumatoid arthritis, targeting these haywire CD4+ T cells could cease the undesired inflammatory response.
Innovative CAR T Cell Targeting
Researchers at the Memphis Veterans Affair Medical Center sought to target the pathogenic helper T cells responsible for rheumatoid arthritis. To accomplish this, the team designed a CAR T cell that autoreactive helper T cells can bind to.
A typical Chimeric Antigen Receptor (CAR) T cell artificially combines an antigen-detecting region derived from antibodies with a signaling domain derived from killer T cells (see Figure 3). Once infused into the blood, the CAR T cells detect antigens on the surface of cancer cells and subsequently eliminate the cancer. As this method does not work against pathogenic CD4+ cells, the researchers developed their own chimeric antigen receptor in response.
Figure 2 illustrates the team’s innovative design. The receptor maintains the same activation and costimulation domains seen in typical CAR T cells. The antibody component, however, is replaced with something new: a synthetic, rheumatoid arthritis-specific protein complex. The complex mimics a specific, rheumatoid associated “flag and pole” (MHCII and antigen) pair that autoreactive helper T cells in mice can recognize. Once bound, the CAR T cell releases chemicals to kill the bound helper T cell.
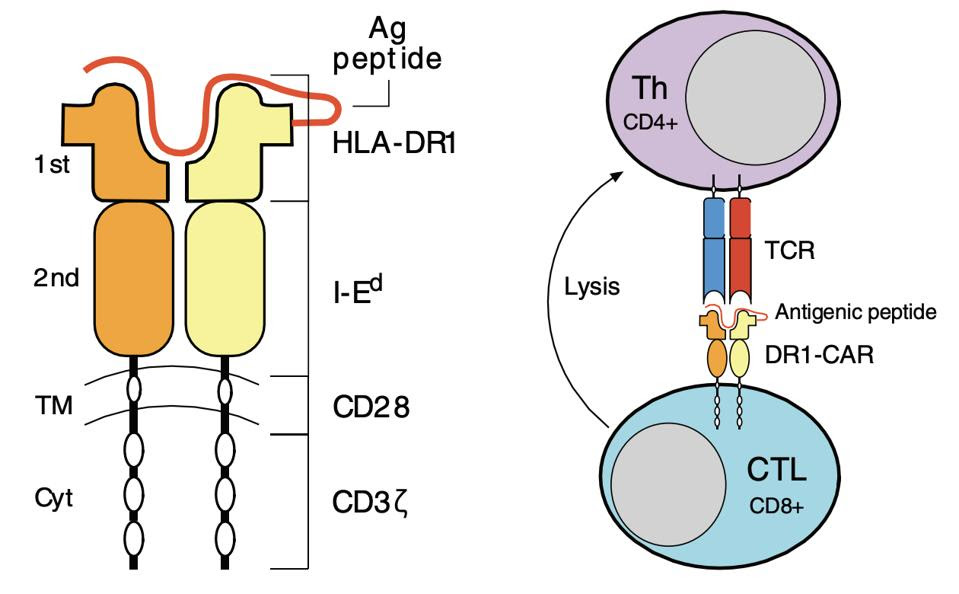
FIGURE 2: This study uses a chimeric antigen receptor which contains CD3ζ T cell machinery alongside CD28 costimulatory molecules. The most notable departure from cancer-based CAR T cell design is the major histocompatibility class II/antigen domain, which enables the cell to interact with pathogenic CD4+ T cells in mice. Abbreviations: CTL, cytotoxic T cell; Th, helper T cell; TCR, T cell receptor.
WHITTINGTON ET AL.
Study Results
The researchers found that the new CAR T cells could indeed be recognized by rheumatoid-associated helper T cells when tested in the lab; the helper T cells could only be stimulated if the corresponding antigen on the CAR T cell was present. The synthetic T cells also successfully eliminated the pathogenic helper T cells.
The researchers then used the CAR T cells on mice immunized with collagen type II (CII). This induced autoimmunity similar to that of human rheumatoid arthritis. The team removed killer T cells from the mice, genetically modified them to express the DR1 chimeric antigen receptor, and reinfused them into the body.
Overall, this new CAR T cell design seemed to effectively eliminate the target CD4+ T cells. The mice treated with the CAR T therapy had significantly reduced helper T cell responses—in some cases completely eliminating the them. As seen in Figure 3, the administration of CAR T therapy also reduced autoantibody responses linked to rheumatoid tissue damage.
In addition, the treatment delayed the onset and severity of rheumatoid arthritis in mice when given early on in disease development. The eventual arrival of arthritis signaled that the CAR T cells, effective as they may be, circulated in the body for a shorter time than expected. The CAR T cell design may need further adjustments to support CAR T cell expansion inside the body.
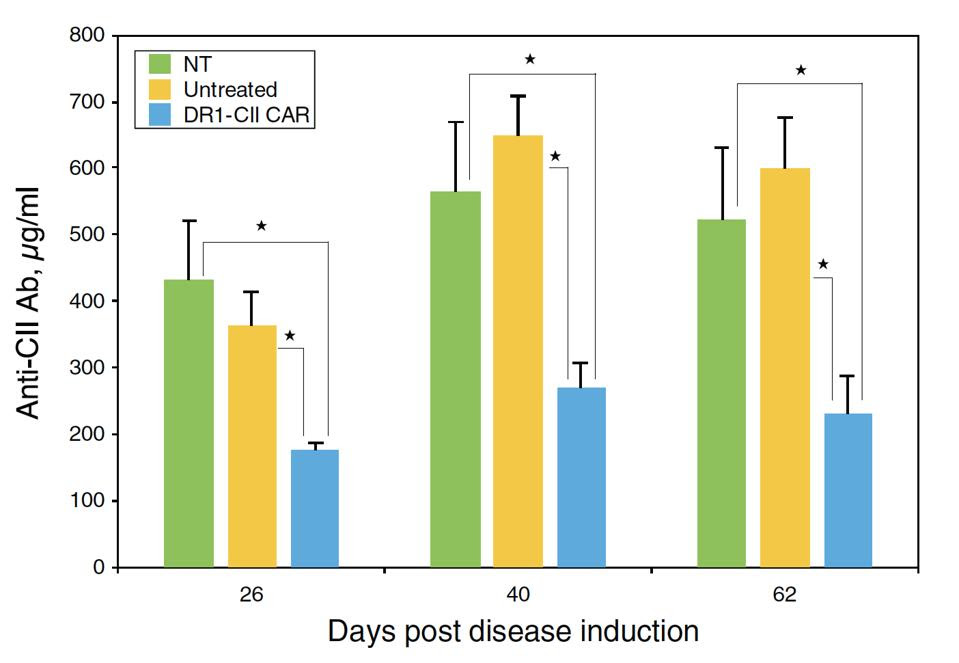
Mice treated with DR1 CAR T cells (in blue) produced fewer autoantibodies
WHITTINGTON ET AL.
Future Directions
This study illustrates that CAR T therapy can indeed translate from cancer to autoimmune treatment. The CAR T cells successfully targeted and eliminated self reactive CD4+ cells, as well as reduced autoantibody production. While the concept proves feasible in mice, the hope is to translate this work for human use. The results broaden the scope of possibility for treating and potentially curing rheumatoid arthritis and other autoimmune diseases.