Progress In Tissue Engineering: Controlling Cell-Cell Signaling
(Posted on Sunday, January 22, 2023)

Cellular adhesion remains a fundamental component to cell-cell communication
GETTY
Cells are the fundamental unit of life. The average human body contains around 30 trillion cells with varying functions and potentials. Communication between cells is central to the body’s ability to form tissues and organs, and to coordinate critical functions throughout the body.
We are now learning the language cells use to communicate with one another in hopes of treating a wide range of diseases, as well as repairing and regenerating tissues. A recent series of studies elucidate new aspects of cellular conversation. Here, we highlight a study which demonstrates the interchangeable nature between the function of cell adhesion molecule receptors and their intracellular plasmic regions.
Understanding Cell Adhesion Molecules (CAMs)
Researchers at the Wendell Lim lab investigated cell to cell interactions formed by cell adhesion molecules. Cell Adhesion Molecules (CAMs) are transmembrane proteins which can mediate cell to cell communication. These molecules allow a cell to bind or “stick” to a nearby cell through a process called cellular adhesion. The organized arrangement of cells connected by this cellular glue contributes to the formation of tissues, while the interaction can spark a signaling cascade within the cell.
Breaking Down the Components
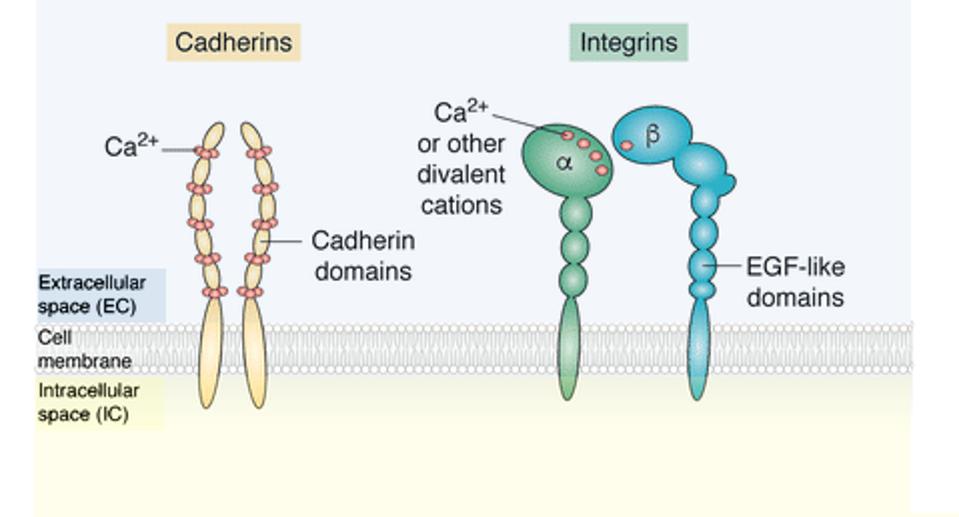
To further understand cell adhesion, it is important to know the three main regions of a cell adhesion molecule. Figure 1 highlights each portion of the protein.
The extracellular domain describes the portion of the protein which lies outside the cell; this part of the protein binds with other cell adhesion molecules nearby. The transmembrane domain penetrates the membrane, while the intracellular cytoplasmic domain lies inside the cell.
During cell adhesion, the extracellular domain binds to another cell adhesion molecule. In response, the transmembrane region rearranges the structure of the intracellular domain—a process called allosterism—to activate intracellular signaling pathways.
Figure 1 also illustrates two major types of cell adhesion molecules: cadherins and integrins. Cadherins must bind with other cadherin molecules to promote cell-cell binding. In contrast, integrins bind with a variety of other adhesion molecules to attach a cell to molecules found in the extracellular matrix (see Figure 2). Cadherins and integrins often work alongside each other to create many forms of cellular communication.
FIGURE 1: Many types of cell adhesion molecules exist. Two examples include cadherins and integrins. Cadherins depend on calcium ions (Ca2+) ions to function, while integrins can utilize a variety of ligands. Important to note are the main regions of cellular adhesion molecules: the extracellular domain, the transmembrane (cell membrane), and the intracellular domain. Abbreviations: EGF-like domains, evolutionary conserved protein domain.
JANISZEWSKA ET AL, 2020.
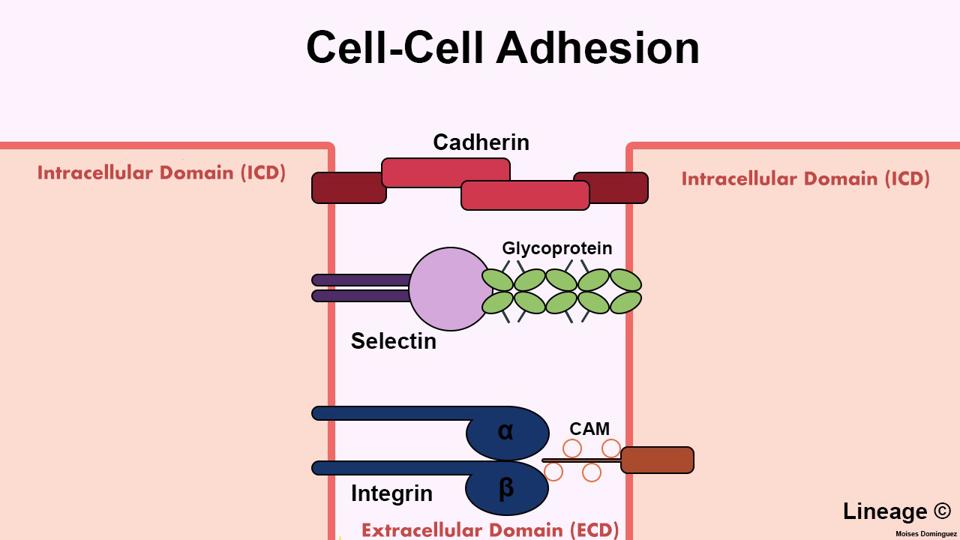
FIGURE 2: Cell Adhesion Molecules (CAM) bind in various ways. Cadherins represent a family of adhesion molecules which only bind to cadherins found on other cells. On the other hand, integrins bind to different types of adhesion molecules to promote cell to extracellular matrix binding. Although not mentioned in this study, selectins are adhesion molecules which bind to sugar molecules on other cells.
LI & DOMINGUEZ, 2019.
Synthetic CAMS—How do they fare?
In this study, the researchers artificially constructed several types of synthetic cell adhesion molecules to better comprehend the underlying rules and nature of cell adhesion molecules as a whole. Modular in design, the team interchanged the internal components—specifically in the transmembrane and intracellular matrix—with a different component in the extracellular matrix to yield varying results.
For the first stage of the study, all of the synthetic molecules shared a green fluorescent protein attached to an α green fluorescent protein nanobody in the extracellular matrix. The inner portion varied between eight different intracellular regions in total, including E-cadherin, Integrin β1, and Neural cell adhesion molecule 1. The team also created a tether—a synthetic molecule with only the green fluorescent protein combo and no intracellular tail—to observe extracellular binding impacts independently. Figure 3 visually compares natural adhesion, the tether and the synthetic adhesion design.
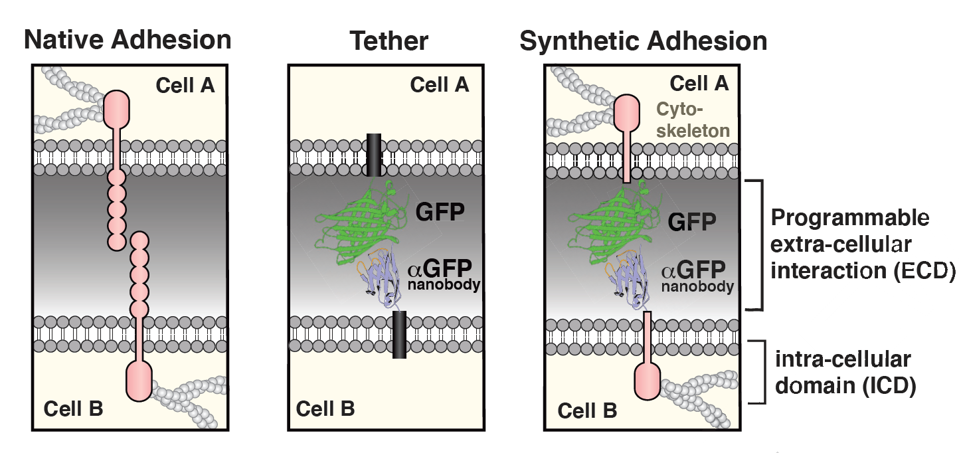
FIGURE 3: Unlike native adhesion, synthetic cell adhesion molecules can be artificially made using different extra and intracellular domains. In this figure, a green fluorescent protein (GFP/αGFP) nanobody in the extracellular domain is paired with variable molecules in the transmembrane/intracellular domains.
STEVENS ET AL, 2022.
Comparable to Native CAMs
To study the efficacy of the new adhesion molecules, the team analyzed the binding interfaces created when using synthetic adhesion molecules to join mice fibroblast cells.
The synthetic cell adhesion molecules perform similarly to their natural complements. Some manufactured cell adhesion molecules formed native-like interfaces despite having an artificial extracellular domain. The other molecules demonstrated a small but enriched interface (see Figure 4).
Tissue Integration
The synthetic molecules not only perform comparably to native adhesion molecules, but also integrate successfully with tissues formed by native cell adhesion molecules. Natural cell adhesion molecules (P-cadherin) interact successfully with synthetic cell adhesion molecules when inserted in a three dimensional tissue model.
The acceptance of synthetic to natural carries large implications when adapted in a multicellular scale. Cell engineering could change the way in which cells are organized and therefore affect tissue building.
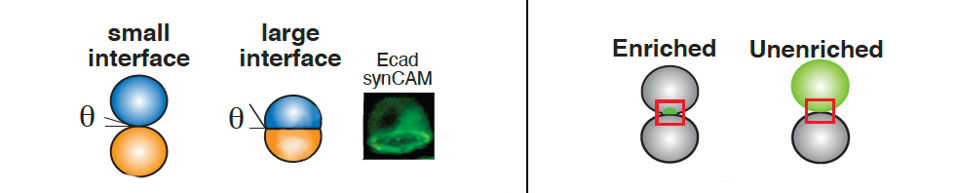
FIGURE 4: The team observed the size (left) and enrichment (right) of an interface formed by their synthetic cell adhesion molecules. They found that the synthetic molecules formed either large, native-like cell interfaces or small but enriched cell interfaces or small but enriched cell interfaces.
STEVENS ET AL., 2022.
Insight on Synthetic Cell Adhesion Molecule Design
The researchers also discovered which domains have a greater impact on cell-cell binding formation. They found that reducing the binding affinity of the extracellular domain decreased the contact angle—a measure of cell-cell surface tension and thus binding—but the interface remained expanded. In comparison, removing the intracellular domain completely disrupts the interface. These observations demonstrate how the extracellular domain independently determines exactly “who” the cell interacts with, and how the transmembrane and intracellular mechanism determines the character of the interface and the cytoskeletal response.
The Future of Cell Engineering
This study deepens current understandings of cell to cell communication. The results show that while it is the extracellular domain that recognizes a stimulus, how the cell reacts to the stimulus depends upon the transmembrane and cytoplasmic regions of the protein; the same intracellular responses occur when the cytoplasmic region is tethered to a heterologous extracellular domain.
The modular nature of these synthetic molecules and their ability to integrate with their native counterparts expands current boundaries of cell engineering. These studies open the exciting possibility to programming novel cell-cell associations for tissue engineering and to control immune and neural cell interactions.