CAR T Cell-Like Therapy To Treat T Cell Leukemia (T-ALL)
(Posted on Saturday, February 18, 2023)
Image of red blood cells and abnormal white blood cells in an acute lymphocytic leukemia cell blood smear, analyzed by microscope at 1000x magnification.
GETTY
As part of our series in cellular therapy, we previously described how CAR T therapy for treatment of B cell leukemia achieved early success in the field. T cell leukemia, however, engages a different part of the immune system and cannot be treated using similar cellular therapies. There is a need for more therapeutic options, as around 15% percent of people with T cell leukemia experience treatment-resistant relapse. Here we discuss a study published in the journal BMJ investigates a promising new approach inspired by CAR T therapy on mouse models.
T Cell Acute Lymphoblastic Leukemia (T-ALL)
T Cell Acute Lymphoblastic Leukemia (T-ALL) comprises up to 15% of acute leukemia cases in children and up to 25% of cases in adults. The condition arises when DNA in a bone marrow stem cell mutates spontaneously. Immature forms of T cells, important white blood cells for immunity, then begin to grow and divide at alarming rates. The abnormal, undeveloped cells ultimately crowd out other healthy white blood cells and impede normal function.
Chemotherapy and stem cell transplants drastically improved prognoses for people with this disease in recent years; the five year event-free survival rate now hovers around 85%. However, if a treatment fails, oftentimes it cannot be repeated due to treatment resistance. This leaves few remaining options for people with relapsed and resistant leukemia. In their study, Jiménez-Reinoso et al. pursue a possible alternative evolved from CAR T therapy.
CAR T Therapy Limitations
Cancer researchers in Barcelona and Madrid, Spain collaborated to determine if a derivative of CAR T therapy can potentially treat relapsed and refractory forms of T cell acute leukemia. Important to this research is an understanding of CAR T therapy, and why it cannot be applied to T cell acute leukemia without alteration.
In CAR T therapy, a practitioner extracts a patient’s T cells, genetically modifies the cells to express new machinery, and infuses the cells back into the patient’s body to fight the cancer. An infused Chimeric Antigen Receptor T cell can specifically recognize and kill all B cells—cancerous or healthy—by identifying a particular biological tag (antigen) found on their cell surface (see Figure 1).
This approach does not translate well for T cell acute leukemia. The major issue lies with the antigen target. An antigen target which attacks all immune cells in the lineage—as CAR T cell therapy often does for all B cells—would cause life-threatening immunodeficiency. The CAR T cells would attack cancerous T cells, cancer-fighting CAR T cells and healthy T cells alike. This is not a problem for patients who receive CAR T therapy to treat B cell cancers; they receive a supply of antibodies to compensate for the diminished amount of B cells in their body. However, a similar therapy to replete T cells does not yet exist.
A Novel Target: Antigen CD1a
A different biological tag is needed—one rarely found on healthy cells, but consistently found on malignant T cells. Jiménez-Reinoso et al. previously made CAR T cells which eliminated cells with an antigen called CD1a. This tag is found in many cancerous T cells and scarcely on any others, making it an ideal therapeutic target. The team used this key discovery to derive a more precise cell therapy.
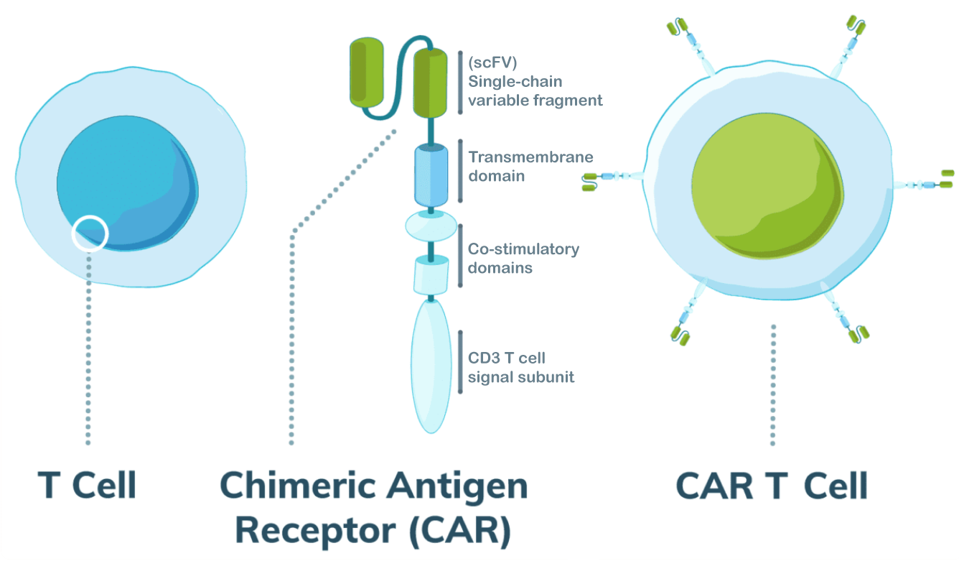
FIGURE 1: Illustration of second generation CAR T cell design. Most important here is the single-chain variable fragment (scFV), which is derived from antibodies to give the T cell improved targeting ability. The fragment detects antigens on the surface of other cells. An anti-CD19+ antibody fragment is used to eliminate B cells for cancers such as B Cell Acute Lymphoblastic Leukemia (B-ALL). The researchers here altered the design to target cortical T cell leukemia cells using an anti-CD1a antibody fragment.
MESOTHELIOMA.COM
The Next Stage: STAb Cell Therapy
A novel target acquired, the researchers created a new therapy: STAb-T immunotherapy, short for Secreting T cell-redirecting Antibodies.
This method modifies T cells to secrete special antibodies. Illustrated in Figure 2, the antibodies target CD1a (the tumor antigen) on one side and CD3 (a common antigen found on T cells) on the other. The team believed that the resulting antibody bridge (see Figure 3) would encourage healthy T cells nearby to target the tumor, as well. This bystander effect doesn’t occur with CAR T cells, which alone engage in tumor elimination.
Results
When tested in vitro and in vivo animal models, the antibody-secreting T cells performed comparatively—if not better—than CAR T therapy.
The team discovered that the bispecific antibodies can successfully bind to neighboring T cells. In cell culture, the bystander effect observed contributed to the stronger killing power observed for STAb cells compared to CAR T cells. The authors note that STAb cells achieve this superior cytotoxicity without releasing as many immune chemicals. This suggests that STAb cells have a reduced risk of a common side effect associated with CAR T therapy called cytokine release syndrome, and thus pose as a safer alternative.
To test the in vivo effect short term the team injected T cell leukemia cells into mice and followed three days later with normal and activated T cells, CAR T cells, or STAb cells. The mice with normal T cells experienced unhampered disease progression, while the mice treated with CAR T and STAb cells displayed similarly controlled levels of leukemia. CAR T cells and STAb cells performed comparably in a longer, two week model as well; the engineered cells could remove and reduce tumor burden when initially challenged and when the disease is artificially relapsed.
STAb T cells may hold a technical advantage over CAR T therapy. In vitro, STAb cells demonstrated killing capacity even at low ratios; the CAR T cells, in contrast, required higher concentrations to elicit significant cytotoxicity. STAb cells may require lower doses to achieve effective results—a factor that could reduce manufacturing costs and allow the therapy to reach more patients once clinically translated.
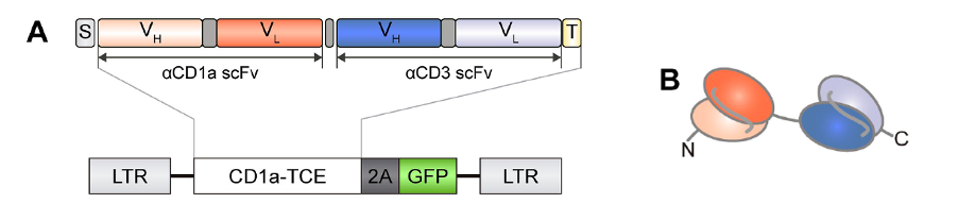
FIGURE 2: (A) Genetic structure of the bispecific T cell-engaging (TCE) antibodies containing an anti-CD1a scFV gene (orange) and an anti-CD3 scFv gene (blue). Abbreviations: S, light chain signal peptide (gray); T, histamine tag (light yellow). LTR, long terminal repeat sequence from lentiviral transfer, GFP, green fluorescent protein. (B) Domain structure of the bispecific antibody. Note the anti-CD1a domain in orange and anti-CD3 domain in blue.
JIMÉNEZ-REINOSO ET AL., 2022.
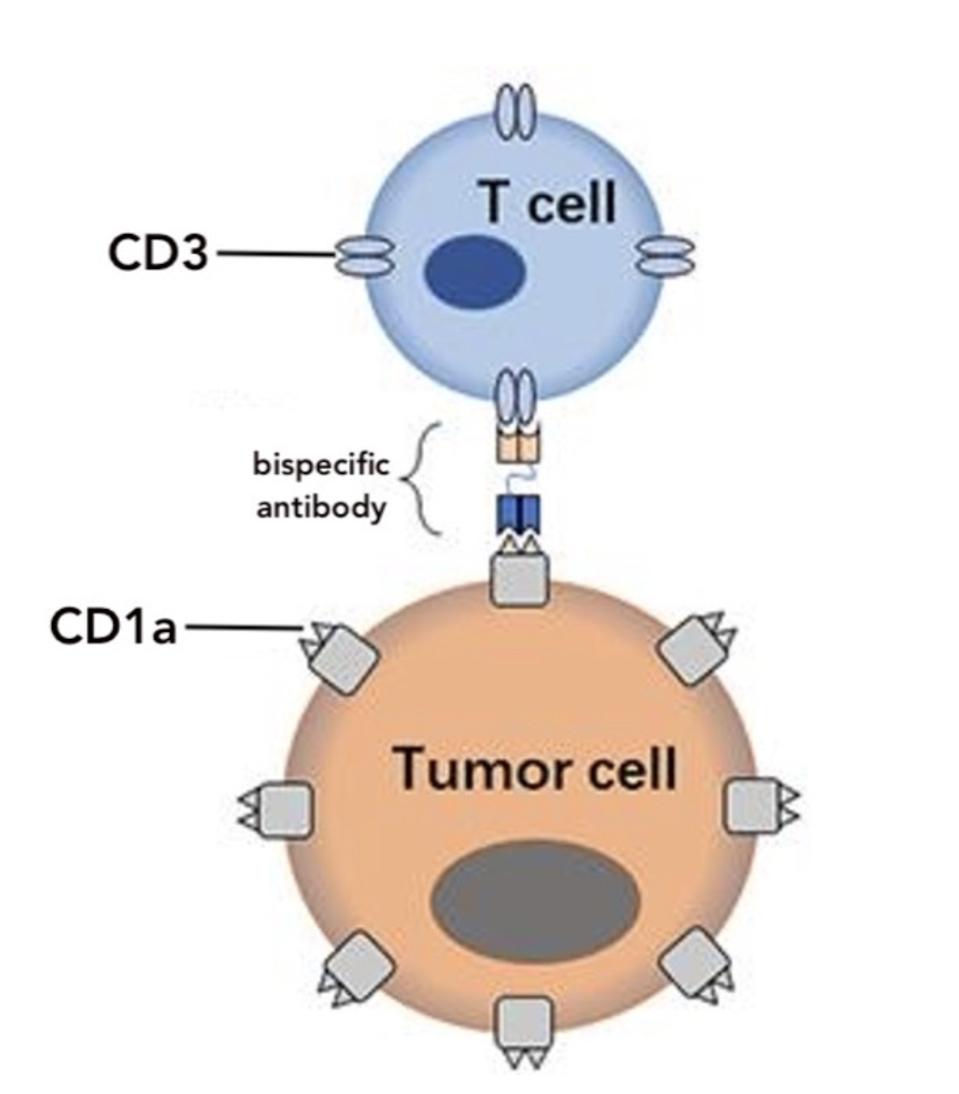
FIGURE 3: The STAb T cell is designed to produce a bispecific antibody. The antibody binds to antigen CD1a on tumor cells and antigen CD3 on nearby T cells like a bridge. This allows unmodified T cells to contribute to antitumor activity.
ASSAY GENIE
Looking Forward
In their study, Jiménez-Reinoso et al. demonstrate the promise of STAb-secreting T cells as an alternative to CAR T therapy. Clinical adaptation would provide a much needed therapeutic alternative for those struggling with treatment resistant T cell leukemia. The paper also exemplifies how bispecific antibodies can bring additional precision to CAR T and similar cell therapies. Another innovative use of antibody bridges can be found here: Researchers Control Cancer Treatment With New Innovation: CAR T Switch(blade).

