Anti-ACE2 Monoclonal Antibodies To Prevent And Treat COVID-19
(Posted on Thursday, March 30, 2023)
Early in the pandemic, monoclonal antibodies proved to be one of the most effective ways to prevent and treat Covid-related disease. Neutralizing antibodies are directed toward specific structures on the spike protein, specifically to the receptor binding domain.
Unfortunately, the virus mutates under selective pressure over time, altering the structure of the receptor-binding domain. The mutated virus retains binding to the surface of lung and other cells, but then they avoid recognition by antibodies. Over time, each of the antibodies, whether singly or in combination, fails to protect against these virus variants.
Researchers Zhang et al. from the Rockefeller and Stanford Universities investigated a different approach. Rather than directing antibodies to the receptor binding domain, their strategy was to develop antibodies that recognize ACE2 on the host cell, to which the virus spike binds.
Here we analyze their findings, which may have the potential to offer an alternative therapeutic pathway for antibody development moving forward.
Generation of hACE2-binding human monoclonal antibodies
To find ACE2 binding monoclonal antibody candidates, Zhang et al. immunized humanized mice models that produce chimeric antibodies consisting of human Fab domains and a murine Fc domain. They were immunized with ACE2 extracellular domains.
Following a 35-day observation period, hybrid B cells, or hybridomas, were isolated from mice sera. Ten candidates were selected that secreted antibodies that impeded SARS-CoV-2 pseudotype infection.
Human anti-hACE2 mAbs broadly inhibit sarbecovirus infection
Six of the 10 chimeric human-mouse antibodies were chosen to be adapted to a human IgG expression for their genetic diversity and binding capacity. All six generated human antibodies blocked a pseudotype SARS-CoV-2 wildtype virus from infecting target cells. Notably, two antibodies were more than 10-fold more potently neutralizing than a previously investigated anti-ACE2 antibody, h11b11.
The group of six also inhibited infection by pseudotype variants, including Beta, Delta, and Omicron BA.1.
The researchers found that the antibodies also inhibited a SARS-CoV-1 pseudovirus, as well as two pangolin and two bat viruses with similar potencies.
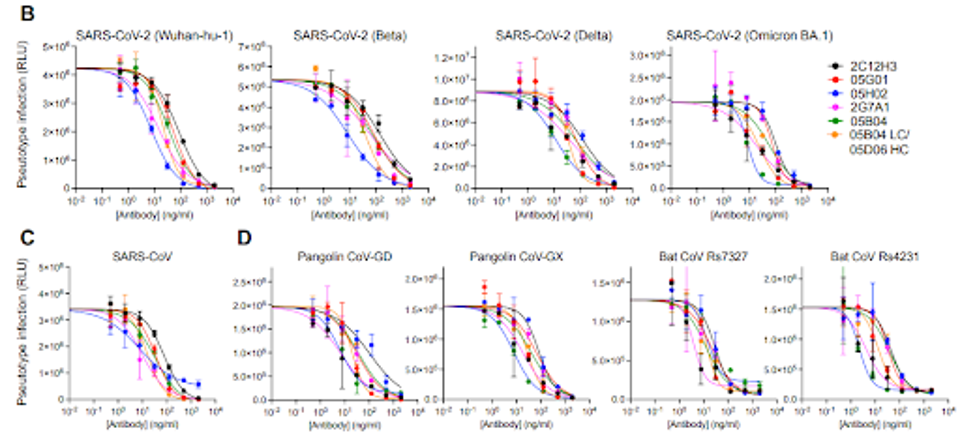
FIGURE 1: Inhibition of HIV-1 based pseudotyped (A) SARS-CoV-2, (B) SARS-CoV-1, or (C) pangolin and bat virus infection by anti-hACE2 mAbs.
ZHANG ET AL.
Vero E6 cells were incubated with the 05B04 antibody candidate ad later introduced to the wild-type live virus. 05B04 also potently inhibited the live BA.1 virus, demonstrating its capability to neutralize a range of SARS-CoV-2 variants.
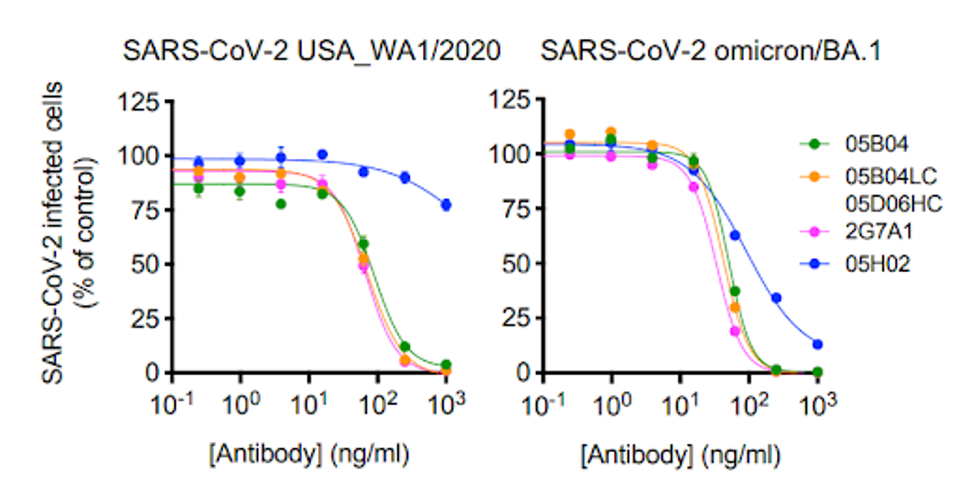
FIGURE 2: Three of four anti-ACE2 monoclonal antibodies overcoming wildtype and BA.1 live virus.
ZHANG ET AL.
Structural analysis of anti-hACE2 mAbs
The ACE2 site on human cells has natural functions and it is crucial the introduction of antibodies does not impact these functions. The anti-ACE2 antibodies must bind an epitope that solely impacts the binding capacity of the SARS-CoV-2 receptor-binding domain and not other cellular enzymatic activity. With rare exception, human genes remain unmutated in regards to ACE2 design, and therefore, it is crucial that the antibody does not overlap the receptor-binding domain and substrate-binding site.
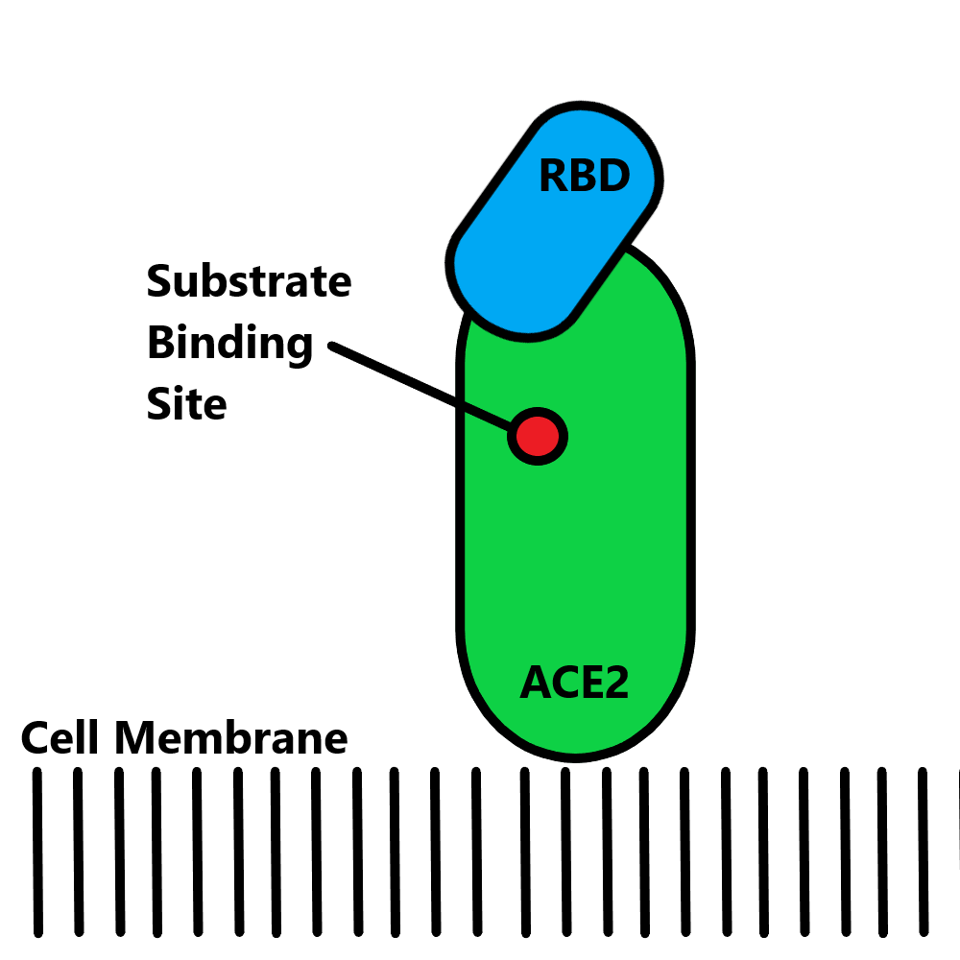
FIGURE 3: ACE2 schematic. The ACE2 (green) is bound by the spike receptor-binding domain (blue), with the substrate binding site (red) denoted. It is crucial that anti-ACE2 antibodies do not overlap the receptor-binding domain epitope and the substra
ACCESS HEALTH INTERNATIONAL
With the aid of cryo-electron microscopy, they found that antibody interactions mimicked favorable binding between SARS-CoV-2 receptor-binding domains and an N-terminal helix of ACE2, a critical juncture for SARS-CoV-2 infection. This molar mimicry enables high binding affinity, despite a smaller binding epitope than the virus.
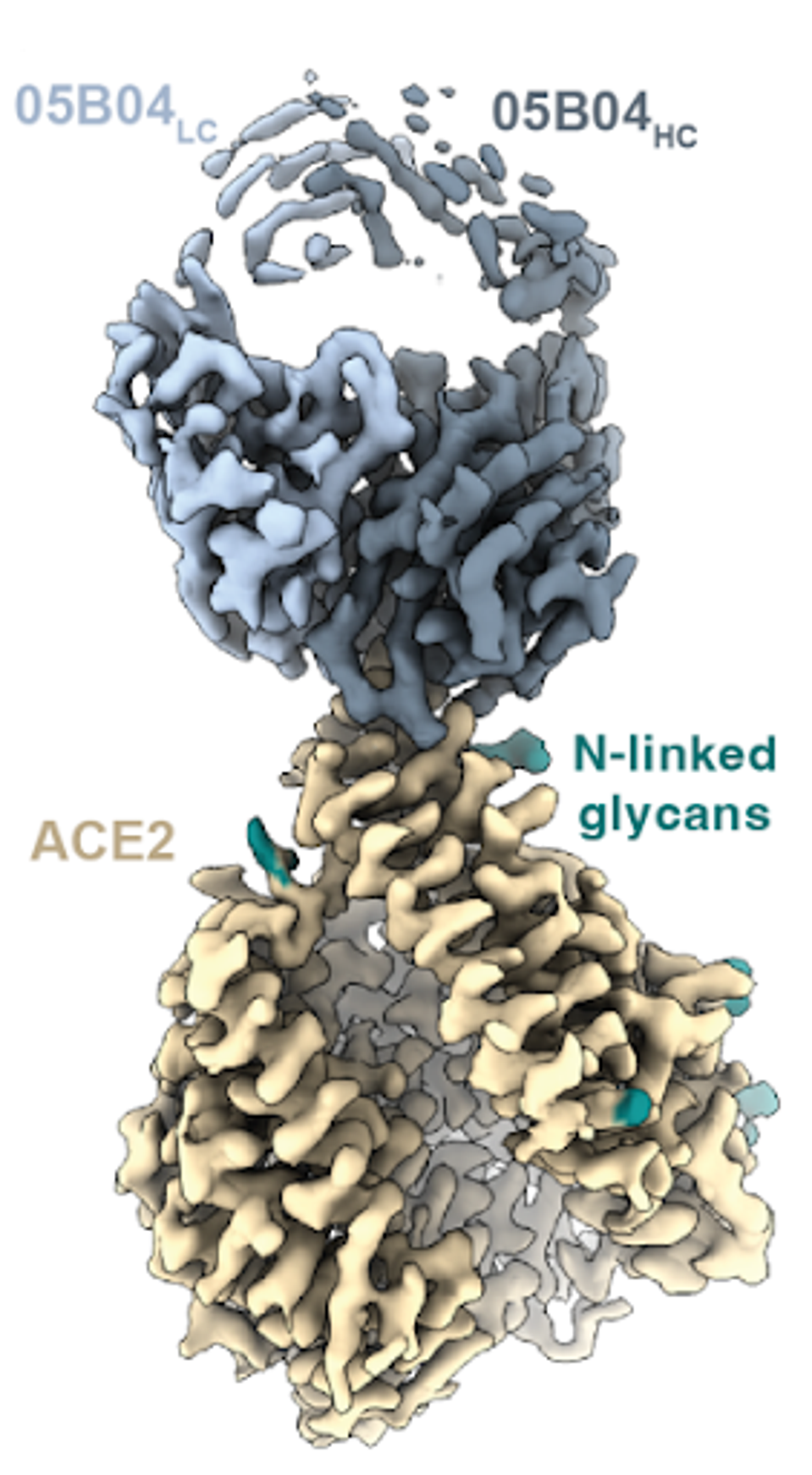
FIGURE 4: Cryo-EM structure of the 05B04-hACE2 complex.
ZHANG ET AL.
hACE2 mAbs protect hACE2 knock-in mice against SARS-CoV-2 infection
In animal models, the anti-ACE2 antibodies continue their positive data points. In mice adapted with a human ACE2, subjects were subcutaneously injected with 250 micrograms of anti-ACE2 monoclonal antibody. There were no observed pharmacokinetic issues in the mice during a 14-day observation period.
Two days later, the mice were introduced to live wild-type SARS-CoV-2. Pre-treatment with the anti-ACE2 antibody reduced lung virus replication to near indetectable levels as compared to the control. Therefore, the anti-ACCE2 antibodies were successful as a prophylactic for SARS-CoV-2 infection, albeit against the wild-type virus.
Discussion
This is a promising new approach to antibody development, though some concerns must be addressed. Firstly, can a high enough concentration of these antibodies be delivered to block enough receptors to be effective against the invading pathogen? Secondly, would blocked receptors interfere with normal cellular physiology? Until extensive human studies are conducted, we cannot know the answer.
Moreover, we have continually underestimated the genetic and structural flexibility of the coronavirus family. For instance, a close relative of the MERS virus binds not to the typical MERS receptor CD26, but rather it uses a different domain to bind to bat and even human ACE2 receptors, albeit using an alternative face.
Given this flexibility, it is conceivable that variants of SARS-CoV-2 may evolve to use not the typical front face of the receptor-biding domain but rather other receptor-binding domain epitopes.
Nonetheless, this is an interesting and promising approach. Another approach that we often describe is the use of broadly neutralizing monoclonal targeting conserved epitopes on the spike protein, a number of which have been discovered and some of which are in clinical development. These strategies may be complementary and antibody cocktails that include ACE2 binding antibodies may be part of the answer following phase one safety and phase two efficacy trials.

