Promising Monoclonal Antibodies For The Treatment Of Yellow Fever Virus
(Posted on Tuesday, April 4, 2023)
Yellow Fever virus is among the most dangerous pathogens circulating today. As many as half of hospitalized patients succumb to disease complications. The disease typically impacts tropical climates, such as central Africa and South America, where mosquitos are heavily present.
However, the Yellow Fever virus has historically been a serious problem for the United States. Throughout the 18th and 19th centuries, Yellow Fever ravaged port cities as far north as Boston as trade in the post-revolution United States reached its full potential. Cities such as New Orleans, Savannah, and Charleston were hit the hardest, accounting for over 100,000 Yellow Fever deaths. Many thousands of Americans were also infected during the building of the Panama Canal.
While recent vaccine advances have limited new infections, effective treatments for those with a Yellow Fever virus infection are nonexistent.
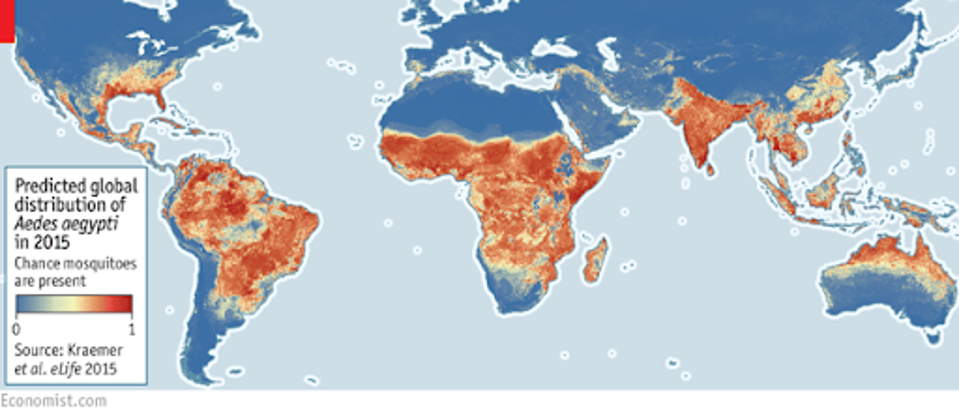
Global distribution of mosquitoes, concentrated between the Tropics of Cancer and Capricorn.
THE ECONOMIST
Yellow Fever virus is estimated to cause 30,000 deaths annually, with 90% occurring in central Africa. The primary transmission method is by the bite of the Aedes or Haemagogus mosquito, with symptoms coming on roughly five days after the initial infection.
The only presently available treatment or prophylactic is the YFV-17D vaccine. The US Department of Health and Human Services recommends a YFV-17D booster every ten years for those traveling to areas affected by the Yellow Fever virus. The vaccine was developed in 1938 at Rockefeller University. Since that time, no other live attenuated vaccine has been created, despite ongoing efforts.
In a recent study by Ricciardi et al., human monoclonal antibodies were isolated from the sera of volunteers vaccinated with the Yellow Fever virus vaccine YFV-17D. The researchers analyzed the isolated antibodies against both in vitro and in vivo viruses, finding that several antibodies sufficiently neutralized virus samples. Here we analyze their findings and discuss the implications on global Yellow Fever virus outbreaks.
Antibody Screening
Ricciardi et al. isolated 489 antibodies from memory B-cells extracted from the sera of over 1,200 Yellow Fever virus vaccinees. They selected 38 candidates based on their high neutralizing capacity.
The antibodies were further selected based on the neutralization of Yellow Fever virus alternate strains, specifically the common isolate, YFV-ES504, associated with the 2016 outbreak in Brazil. Just 16 of the 38 neutralized the Brazilian isolate with a sufficient IC50 value. The final antibodies selected were two that do not compete in binding, suggesting they bind different epitopes. These maintained the strongest Brazilian isolate neutralization at low concentrations.
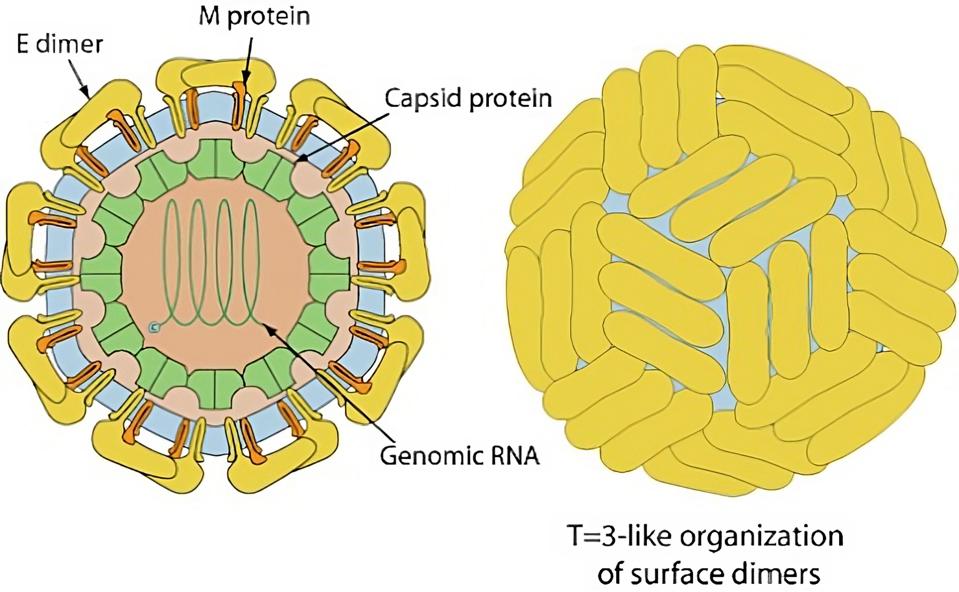
FIGURE 2: Yellow Fever virus virion. The antibody target is the Envelope, or E dimer.
VIRALZONE
The two antibodies, MBL-YFV-01 and MBL-YFV-02, also effectively neutralized three other varying Brazilian isolates, YFV-4408-1E, YFV-RJ155, and YFV-GO09, all associated with different Yellow Fever virus outbreaks. Again, the two independently neutralized all three isolates.
Therapeutic mAb administration protects hamsters and macaques from pathogenic YFV infection
Ricciardi et al. next infected Syrian golden hamsters with adapted Yellow Fever virus to analyze the in vivo efficacy of MBL-YFV-01 and MBL-YFV-02. Three days after infection, the hamsters were administered a single antibody dose of either MBL-YFV-01 or MBL-YFV-02.
Both antibodies significantly improved hamsters’ survival rate, with 100% of those treated with MBL-YFV-01 overcoming the disease. In contrast, only four of 15 in the control group survived their disease course.
Additionally, health markers such as weight fluctuation and liver disease found that those treated with monoclonal antibody candidates survived their symptoms and experienced a wholly reversed disease course, indicating strong protection from death and severe illness.
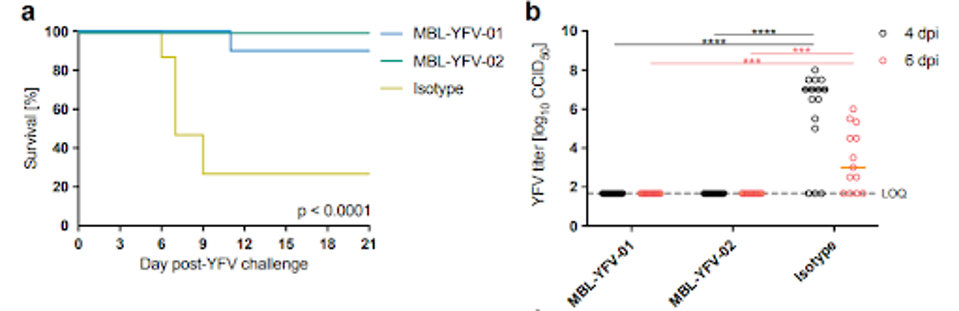
FIGURE 3: Neutralizing monoclonal antibody administration protects Syrian golden hamsters from lethal YFV infection. (a) Survival rate of control vs two antibody candidates; (b) titer reduction of YFV in control vs two antibody candidates.
RICCIARDI ET AL.
In further in vivo testing, Ricciardi et al. infected macaques with the adapted Yellow Fever virus. Following the same pattern as the Syrian golden hamsters, macaques treated with MBL-YFV-01 or MBL-YFV-02 on day two survived three weeks following infection, compared to control macaques succumbing to the disease on day five of infection. All but one macaque had no detectable Yellow Fever virus in their sera 21 days post-infection., suggesting MBL-YFV-01 and MBL-YFV-02 are strong candidates as anti-Yellow Fever virus treatments.
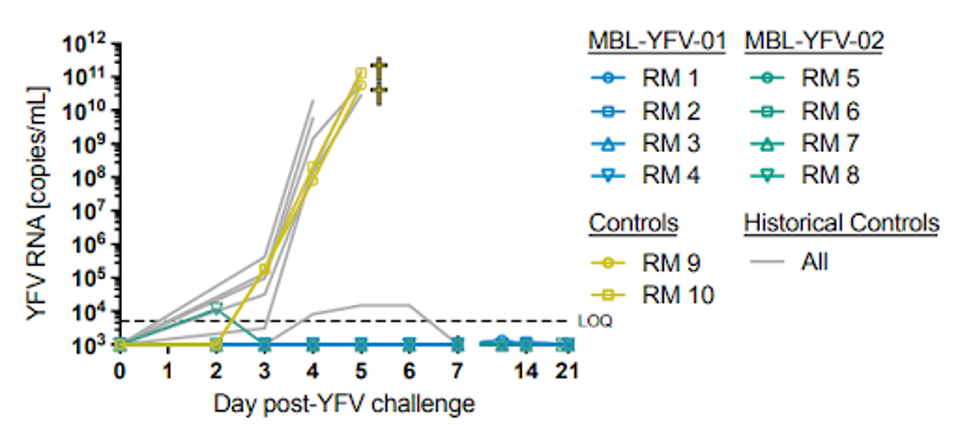
FIGURE 4: Detectable RNA is the sera of YFV-infected macaques treated with monoclonal antibodies (blue and green) vs a control (yellow).
RICCIARDI ET AL.
Discussion
Recent advances in antibody technologies have enabled new and exciting treatments for pathogens previously untreatable. High-mortality diseases such as Yellow Fever, Dengue, Zika, and Ebola have ravaged poorer communities without access to high-quality healthcare. While vaccines effectively prevent some of these diseases, treatment methods are often limited to general medical support, which is often limited in these communities.
MBL-YFV-01 and MBL-YFV-02 represent a step forward in treatment for the Yellow Fever virus and high mortality diseases in general. To understand potential protection against current and future variants of the Yellow Fever virus, future studies must include structural analyses of the antibodies to understand the exact binding epitope to the virus. While further human safety and efficacy are necessary to consider these two antibody candidates a success, their initial in vitro and in vivo data show great promise. We can only hope these antibodies soon advance to clinical trials before helping those in desperate need of an effective treatment.

