A New Generation Of Flexible And Controllable CAR T Therapies
(Posted on Sunday, May 28, 2023)

Chimeric Antigen Receptor T cell (CAR T) therapy is one of the exciting new modalities of cancer treatment. The therapy modifies a patient’s own immune cells to treat specific tumors. This is traditionally accomplished by bioengineering hybrid receptors and attaching them onto a set of killer T cells taken from the patient. CAR T therapy has yielded exciting results for those with certain blood cancers, but as with many early, first wave cancer therapies, there’s substantial room for improvement.
One previously discussed solution used a peptide neo-antigen (PNE) adaptor administered through an injection to potentially reduce adverse effects. Reducing or halting the adaptor dosage prevented the CAR T cells from overreacting. Here, we discuss a new and distinct adaptor system published in the journal Nature Communications which also holds promise.
Why Use Adaptors?
Chimeric Antigen Receptor T cell (CAR T) therapy is an effective cell treatment against certain blood cancers. However, it also carries distinct and intrinsic limitations.
Single Antigen Targeting
One major limitation of traditional CAR T therapy is that the engineered cell can only target a single antigen. To treat multiple myeloma, for example, the CAR T cell binds to BCMA found on the myeloma cell surface. It would be wonderful to integrate multitargeting instead, thus expanding the therapy’s potential applications.
Multitargeting is a potential solution to antigen escape, a phenomenon that occurs when tumor cells in patients in remission express less of the target antigen over time. When this happens, CAR T cells can no longer recognize their enemies, and cancer returns as a result. Rather than repeating the already expensive and timely procedure from scratch, engineering a multitargeting CAR T cell right from the start would allow the therapy to remain effective for longer. This would particularly benefit patients with solid tumors. These tumors express multiple varied antigens on their cell surface, rendering traditional CAR T therapy ineffective.
Lack of Control
Another common issue is lack of control. Sometimes, CAR T cells elicit an overly robust immune response and trigger adverse effects such as cytokine release syndrome or neurotoxicity. Although a weaker CAR T response could prevent these effects, apart from adjusting the infusion dosage, there is no way to precisely control how the CAR T cells react once inside the body. There is, as a result, a begging need to be able to turn the therapy on and off.
Adaptor Has Potential to Overcome Current Limitations
Enter the “universal” adaptor. With this system, the CAR T cell does not bind directly to the tumor antigen as usual. Instead, the cell binds to an antibody adaptor, and the antibody adaptor binds to the tumor cell. Changing or including multiple targets can be achieved by infusing several different kinds of antibodies at once or sequentially. Additionally, altering the adaptor dosage can control the nature of the immune response.
Designing SNAPtag — a Universal Adaptor System
In their study, Ruffo et al. designed a universal adaptor using engineered enzymes called SNAPtag. Although this adaptor demonstrated potential beyond CAR T therapy, here we focus on progress specific to CAR T cells.
Making the Adaptor System
The universal adaptor is made of two fused parts: an antibody and a tag (Figure 1). The antibody end binds to a specific antigen, while the tag on the other end binds to the CAR T cell; this tag is made from a synthetic molecule called benzylguanine (BG).
As illustrated in Figure 2, this adaptor requires a unique kind of chimeric antigen receptor. While the intracellular and transmembrane regions remain the same, the extracellular region adopts a new molecule instead of the usual single chain variable fragment. Importantly, this new, engineered protein attaches strongly via irreversible covalent bonding to any molecule with benzylguanine (Figure 3). Conceptually this means that, so long as the benzylguanine tag is present, a variety of antibodies can be used in this universal adaptor system.
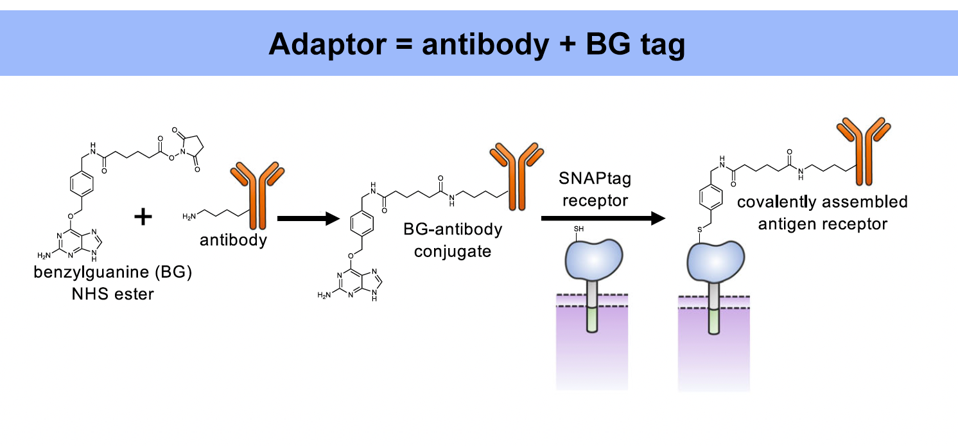
FIGURE 1: The universal adaptor is made by fusing an antibody to a benzylguanine motif (BG). This BG-antibody conjugate bonds covalently to the chimeric antigen receptor to elicit a response.
RUFFO ET AL., 2023.
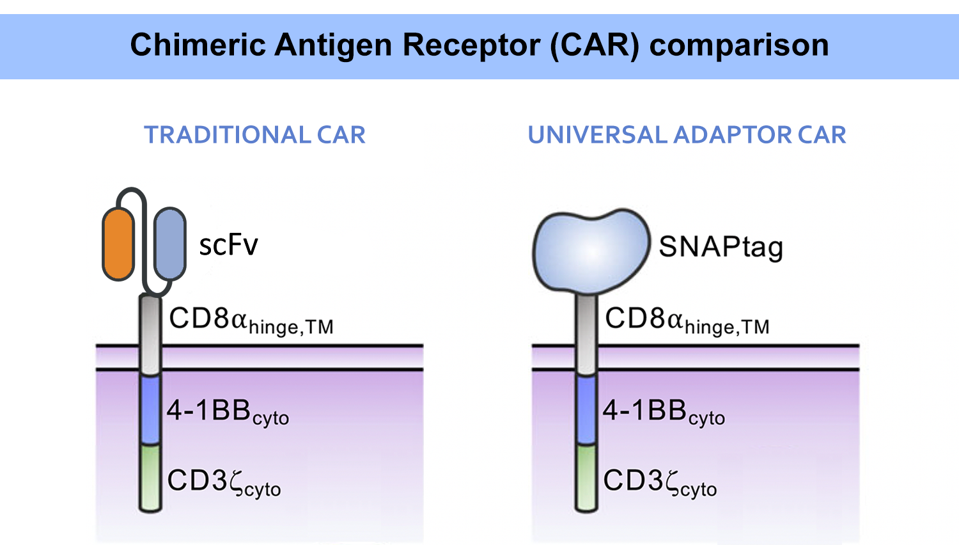
FIGURE 2: A traditional CAR T cell typically uses a fusion antibody (single chain variable fragment, scFV) in its extracellular domain. A different design must be used to work with a universal adaptor. While the adapted CAR T cell contains similar intracellular components, the extracellular domain wields a new protein: SNAPtag. Abbreviations: TM, transmembrane; cyto, cytoplasm.
RUFFO ET AL., 2023.
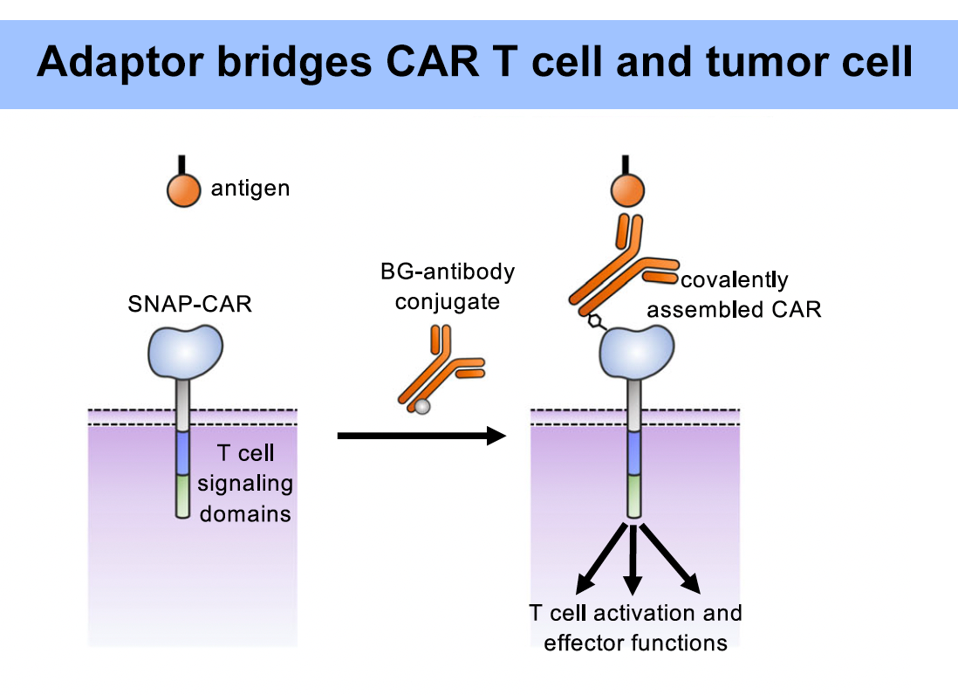
FIGURE 3: The new molecule covalently binds to the universal adaptor, and the adaptor binds to the target antigen. This activates the CAR T signal cascade.
RUFFO ET AL., 2023.
How Does the Adaptor Work?
The presence or absence of the universal adaptor allows a single pool of T cells to complete several functions. The strength of the therapy can grow stronger or weaker depending on the adaptor concentration. Administering several types of antibodies (with the same tag) allows the T cells to target several tumor antigens at once (Figure 4). The antigen target can also be switched by sequentially administering different antibodies. The therapy can be stopped by flooding the system with the tag alone—essentially creating an off switch; without the complete adaptor, the CAR T cell binds to the tag without sparking a response.
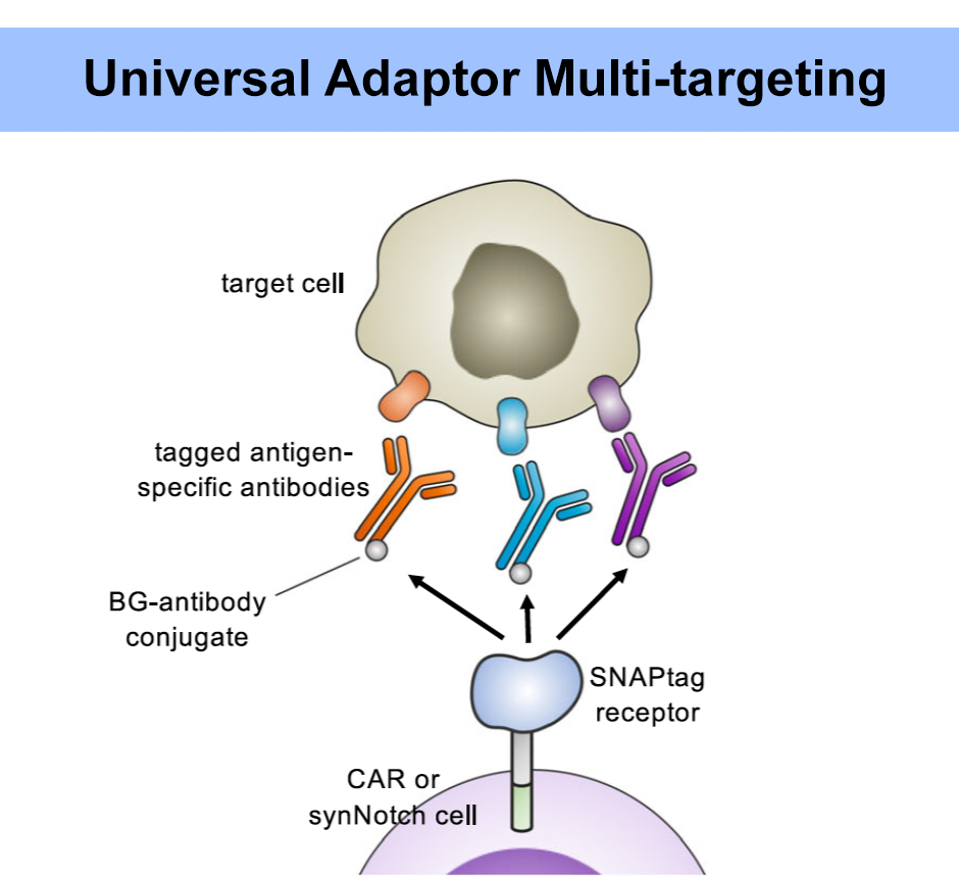
FIGURE 4: The SNAPtag universal adaptor should allow for multiple antigen targeting. The chimeric receptor can respond to different antibodies bearing the same tag. This concept would be particularly beneficial for avoiding antigen escape and for treatment of solid tumors, which notoriously express a variety of antigens on their surface.
RUFFO ET AL., 2023.
Proof-of-Concept in Mouse Models
How might this adaptor and CAR T cell pairing fair therapeutically? To test this, mice were injected with anti-HER2 tumor cells to initiate a tumor graft. After four days, the mice underwent in vivo imaging before receiving one of four treatments: HER2 adaptor alone, SNAP-CAR T cells alone, SNAP-CAR cells with an anti-HER2 adaptor, or antiHER2 CAR T cells (a traditional CAR T cell with a single chain variable fragment). The adaptor injections were given every three days—totaling six injections—while the CAR T infusions were only given once.
Previous testing revealed that the adapted CAR T cells would require antibody supplements to encourage the T cell engraftment. The authors posit that adapted T cells lack antibody protection in these mouse models, and are prone to attack from innate immune or stromal cells. In response, an intravenous antibody supplement was given with the initial SNAP-CAR T cell infusion and any following adaptor injections.
Antitumor Effect of SNAP-CAR T Cells
The mice underwent in vivo imaging every five days until Day 40 or Day 60. The imaging showed rapid tumor growth for the mice treated with the adaptor alone or the SNAP-CAR T cells alone. In comparison, the mice treated with traditional CAR T therapy or the adapted CAR T cells demonstrated significant tumor growth inhibition. By Day 60, the majority of mice in this group showed zero signs of tumor growth.
The researchers repeated this experiment with a new antigen target: CD20. Similarly to before, the mice were inoculated with tumor cells and categorized into four distinct treatment groups: anti-CD20 adaptor only, SNAP-CAR T cells only, SNAP-CAR T cells with the adaptor, and traditional SNAP-CAR cells. The mice with adaptor or SNAP-CAR T cells only experienced rapid tumor growth. However, unlike previously, the remaining mice exhibited partial tumor growth and tumor relapse. This likely is not the result of faulty CD20 targeting; the tumor cells lost CD20 expression over time and therefore could avoid CAR T cell detection.
Future Directions
A universal adaptor extends the use of a single plug and allows it to be used around the world. Similarly, universal adaptors provide a possible solution to CAR T therapy’s many restrictions. This study highlights a particularly versatile design and its ability to shrink tumors in preclinical models. But this universal adaptor has more to offer. In our next installment, we describe how this platform can be combined with a different cell treatment called SynNotch—a duo which may prove even more customizable than its CAR T therapy alternative.

