To Kill And Kill Again: How Cytotoxic T Cells Attack Target After Target
(Posted on Tuesday, June 20, 2023)
Originally published on Forbes on June 15, 2023.
Killer T cells (blue) attack cancer cells (yellow). Recent research reveals key insights into how a
GETTY
Cytotoxic T cells, also known as the “killer” T cells, provide the foundation for cell-based CAR T therapy. The therapy borrows and enhances the T cell’s ability to kill multiple targets in rapid succession. This way, the T cell destroys target after target, thoroughly wiping out any cancer cells it encounters.
This deceptively simple action hides unseen complexity. The ability to swiftly stop cell signaling, detach from the target and pivot to another cell undoubtedly requires precise control—yet, how the cytotoxic T cell accomplishes this feat is not well understood. Research published in the journal Science sheds light on this crucial immune mechanism. Using high-resolution three dimensional imaging, the researchers at Cambridge University discover an underlying process that enables a single T cell to eliminate several target cells.
T Cell Receptors
How do cytotoxic T cells kill? The T cell receptor (TCR) itself is made of two proteins, typically an alpha chain and a beta chain; this portion of the complex recognizes and attaches to particular proteins on the target cell. Once bound, the receptor activates a signaling cascade in the remaining cluster of CD3 chains (Figure 1) to eliminate the target.
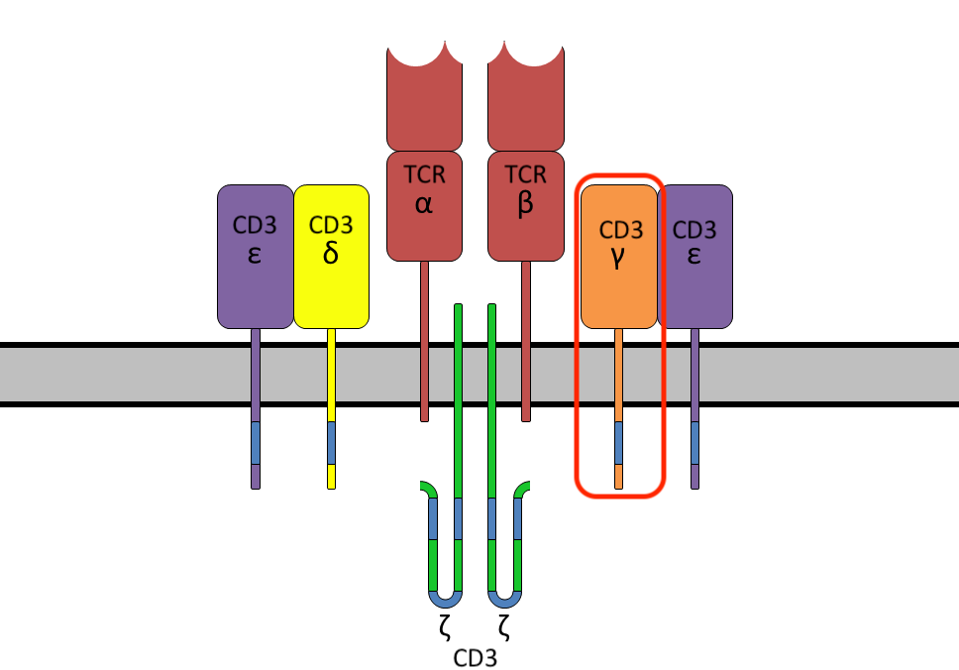
FIGURE 1: T cell receptor (TCR) protein complex, composed of the T cell receptor alpha and beta chains and a cluster of six CD3 chains. In this study, the CD3γ protein is tagged to trace receptor movement when binding to a target cell.
WIKIPEDIA
Prior research suggests that this activation can also downregulate T cell receptor expression on the cell surface. In other words, the same switch to initiate killing can be used to slow down killing activity. Here is where the mystery lies. Does downregulation occur through endocytosis, the process of encircling the receptor with cell membrane and dragging the bubble into the cell? Or is the expression controlled through exosomes or ectosomes, membrane sacs which travel out of the cell? The answers to this question may help us understand how the cell slows down and detaches from its single cell onslaught before moving to the next target.
Study Design
In their study, Stinchcombe et al. use electron microscope tomography to take 12 detailed snapshots of killer T cells interacting with cell targets. During this process, CD3γ proteins were tagged and successfully colocalized with native CD3ε in the cytotoxic T cells. High definition, three dimensional images of the immune synapse—the meeting point between the killer T cell membrane and target membrane—were taken and transformed into three dimensional models. Focus was placed on clusters where T cell receptors were engaged with target protein and killer T cell and target membranes were closely juxtaposed.
Result: Ectocytosis
Although prior literature suggests that T cell receptors are downregulated through endocytosis, three dimensional imaging implies the opposite. As seen in Figure 2, the tomography revealed that around 98% of tagged T cell receptors budded outward from the killer T cell’s edges via right-side out microvesicles called ectosomes. The ectosomes either bubble out or completely detach from the T cell membrane. In contrast, very few tagged cell receptors were internalized by the killer T cell. The results suggest that ectocytosis, not endocytosis, is the key player in T cell receptor downregulation.
This process likely occurs independently from the release of killer granules out of the T cell. In cytotoxic T cells which cannot release potent killing chemicals, ectosomes could still be found budding across the immune synapse to target cells. A significant cytotoxic granule, protein granzyme B, was not found in any ectosomes, as well.
What exactly happens to these vesicles? The receptor-filled sacs could be found deep within the target cell. Untransfected killer T cells performed similarly. This suggests that, once budded, the ectosomes are taken up into the dying target.

FIGURE 2: Three dimensional model of the immune synapse between a killer T cell membrane (magenta) and its target. The image demonstrates how the tagged CD3 chains (green) bud out of the T cell membrane and into target cell space. Abbreviations: CTL, cytotoxic T cell lymphocyte; CD3γ-APEX, CD3γ with an electron microscopy tag (engineered pea ascorbate peroxidase).
JANE C. STINCHCOMBE ET AL., ECTOCYTOSIS RENDERS T CELL RECEPTOR SIGNALING SELF-LIMITING AT THE IMMUNE SYNAPSE. SCIENCE 380,818-823(2023). DOI:10.1126/SCIENCE.ABP8933
Role of Ectocytosis
The team analyzed the possible roles of ectocytosis in T cell serial killing. They posit that the mechanism serves three main purposes. First, ectocytosis stops cell signaling by downregulating T cell receptors at the immune synapse. The number of tagged T cell receptors at the cell membrane decreased as the ectosomes with receptors increased. This reduction is needed to slow or halt cell signaling with the current target.
Next, the target needs to be detached. Ectocytosis benefits this arena, as well. Originally tight contacts between killer T cell and target membrane were lost where ectosomes bubbled from the T cell surface, according to tomography and conventional electron microscopy analyses. Additionally, receptor signal strength increased as ectocytosis and detachment of T cells from targets escalated. Together, these results suggest ectocytosis encourages T cell detachment.
Ectocytosis can also provide a self-limiting function. Self limitation refers to controlling the intensity and duration of the cell’s signaling. Excessive T cell signaling can cause problems in its own right. The ectosome buds and resulting cell detachment prevent the T cell from sustaining cell signaling for too long. The concentrated destructive power is therefore redirected to multiple new targets instead of a single target.
Implications
The new data from Stinchcombe et al. enrich our current understandings of killer T cell actions. Through ectocytosis, the cytotoxic T cell can terminate cell signaling and detach from its current target; the result is a disengaged immune cell ready for a new target. This special feature allows a single T cell to kill not just once, but to quickly eliminate threat after threat. Cancer immunotherapies such as CAR T therapy unwittingly benefit from this fascinating mechanism, and could potentially be improved using this base knowledge.