New Treatment Gives Hope to Chronic Hepatitis D Patients
(Posted on Tuesday, July 11, 2023)
This story was originally published on Forbes on July 6, 2023.
This story is part of a larger series on viroids and virusoids, small infectious RNAs. It is also the seventh installment in a series on hepatitis D virus, a virusoid-like pathogen that causes serious human disease. You may read the others on Forbes or www.williamhaseltine.com.
Chronic hepatitis D is the most severe form of viral hepatitis. Although it leads to similar complications as other viral hepatitis infections —scarring of the liver, liver cancer, and in extreme cases, full liver failure and death— it does so far more quickly: generally those suffering from chronic hepatitis D infection experience liver failure within five to ten years of initial infection, and 15% of people will experience liver failure as quickly as one or two years. Despite the severity of hepatitis D infections, many patients remain asymptomatic for an extended period. By the time you realize something is amiss, the disease has already put down deep roots.
Up until recently, the only treatment for hepatitis D consisted of prevention. As a virusoid-like pathogen, hepatitis D virus depends on a helper virus for entry into, and transmission between, cells; block the helper virus and you block hepatitis D. Thus, vaccination against hepatitis B virus —the helper virus— also offers protection against hepatitis D virus. Now, a new pharmaceutical treatment called bulevirtide has entered the scene. What follows is an overview of this novel drug, including its mechanism of action and real-world trial results.
How Does It Work?
To understand how bulevirtide works, you have to understand how hepatitis D enters host cells. Since hepatitis D is a virusoid-like pathogen, it does not have an envelope or surface proteins of its own. An envelope protects the genetic material of a virus from damage and helps it evade recognition by the immune system. Embedded in the envelope you find surface proteins, also known as glycoproteins. These proteins act as harpoons of sorts, allowing the virus to bind to the surface of target host cells. From there, the genetic information of the virus is absorbed into the cell and the virus can begin replicating.
If hepatitis D virus lacks both of these crucial components, how does it infect cells? Well, it borrows the envelope and surface proteins of hepatitis B virus (Figure 1). Wrapped up in the jacket of another, it can successfully infiltrate host cells. This means the mechanism of entry for both hepatitis D virus and hepatitis B virus is largely identical, since they share the same envelope and surface proteins.
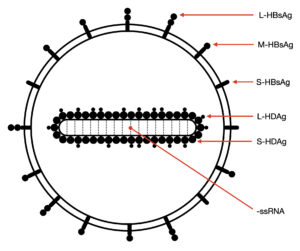
FIGURE 1. Illustration of a hepatitis D virion. Abbreviations: ssRNA, single-stranded RNA; S-HDAg, small hepatitis D antigen protein; L-HDAg, large hepatitis D antigen protein; S-HBsAg, small hepatitis B surface antigen; M-HBsAg, medium hepatitis B surface antigen; L-HBsAg, large hepatitis B surface antigen. SOURCE: Ypna, CC BY-SA 4.0 <https://creativecommons.org/licenses/by-sa/4.0>, via Wikimedia Commons
When a hepatitis D virion approaches a human liver cell, a region of the hepatitis B surface antigen attaches to tendril-like proteins that extend from the surface of the host cell. These tendrils, formally called heparan sulfate proteoglycans (HSPGs), pull the virion closer to the cell membrane. Once at the membrane, the large hepatitis B surface antigen hijacks and tightly binds sodium taurocholate cotransporting polypeptide (NTCP), a receptor on the surface of the liver cells. Usually, NTCP regulates the uptake of bile acids, which help with digestion by breaking down fats into fatty acids, but the large hepatitis B surface antigen subverts this receptor to suit its own needs.
In addition to NTCP, epidermal growth factor receptor (EGFR) is also a key player. This receptor interacts with NTCP and triggers endocytosis, a process by which the cell membrane folds in on itself and forms a small bubble, called a vacuole, around the HDV-NTCP-EGFR complex (Figure 5). The vacuole carrying the complex is brought into the cell. A specific section of the hepatitis B surface antigen then acts as a translocation motif (TLM); essentially, a set of instructions that allow the hepatitis D viral particle to escape the membrane bubble through which it was brought into the cell. Escaping the vacuole lands the particle in the cytoplasm of the cell, one step closer to where it wants to be: the nucleus. Once in the nucleus, hepatitis D virus can begin replicating.
Bulevirtide works by mimicking the surface protein of hepatitis B virus, thus competing with the protein for the sodium taurocholate cotransporting polypeptide (NTCP) receptor. If bulevirtide binds the receptor first, then the hepatitis B surface proteins cannot attach themselves to the cell membrane, and by extension, cannot enter the cell — only one item can bind to the receptor at a time. By blocking cell entry, bulevirtide blocks replication as a whole.
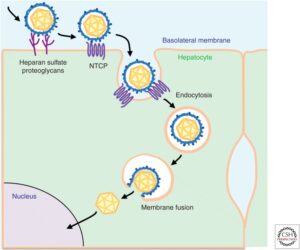
FIGURE 5. Schematic representation of the hepatitis B virus entry pathway. Since hepatitis D virus uses the surface proteins of hepatitis B, cell entry for the two is understood to be the same. Only once in the cytoplasm does hepatitis D replication become HBV-independent. SOURCE: Koichi Watashi and Takaji Wakita 2015 https://doi.org/10.1101%2Fcshperspect.a021378
How Well Does It Work?
Real-world studies represent the gold standard for gauging how well a pharmaceutical product works. So far, bulevirtide has proven up to the challenge, with positive results across three different real-world studies.
The first of these was performed by researchers at the Center for Liver Disease at the University of Milan. Degasperi et al. treated 18 patients with 2mg a day of bulevirtide for a period of 48 weeks. All of the patients displayed signs of liver cirrhosis and clinically significant portal hypertension, elevated pressure in the veins that lead to the liver. For five of the patients, hepatitis D RNA levels became entirely undetectable during the course of treatment. For all but two of the patients, there was a clear virological response accompanied by a decrease in hepatitis D RNA. Levels of aspartate aminotransferase and gamma-glutamyltransferase —two key markers of liver health— also improved markedly. The only side effect experienced by the patients was an asymptomatic increase in bile acid, which is to be expected since bulevirtide targets the receptor in charge of bile acid regulation. None of the patients discontinued treatment.
The same group of researchers released a case report on a three-year course of bulevirtide treatment. As before, this was a patient who already showed signs of hepatitis D-related organ damage, including liver cirrhosis and abnormal veins in the lower part of the tube running from the throat to the stomach, known as esophageal varices. Following continuous treatment for three years, levels of hepatitis D RNA and hepatitis D antigen protein dropped to undetectable levels. Again, all relevant markers of liver health showed significant improvement, including: normal liver enzymes, preserved synthetic function, normalization of immunoglobulin G and gamma-globulins, and regression of the esophageal varices. Both the virological response and the improved liver health were maintained for the duration of a 72-week follow-up period during which the patient did not receive any bulevirtide. Barring any later flare ups, the three-year treatment essentially cured the patient of chronic hepatitis D.
Finally, scientists at the Hannover Medical School gathered data from 114 patients treated with 2mg of bulevirtide. A clinically significant decrease in hepatitis D virus RNA was observed in 76% of patients. The mean time to virologic response was 23 weeks. Again, there was noticeable improvement in liver function, even in those patients that did not achieve a significant virologic response.
Takeaways
The development of bulevirtide is a major step forwards; for the first time since the discovery of hepatitis D virus in 1977, patients have a treatment available to them that effectively challenges chronic infection. Not only does bulevirtide suppress the virus to undetectable levels, it also helps heal the liver damage associated with infection.
Although a victory, we would do well to remember that it is better to be overprepared than underprepared. Yes, having a functional drug to combat chronic hepatitis D is cause for celebration, but redundancy is crucial. We need to expand our quiver to ensure we stay one step ahead. This is best done by diversifying our lines of attack, making it harder for hepatitis D virus to evade treatment. For example, novel research indicates that hepatitis D may also be transmitted by different helper viruses. Since bulevirtide works by interfering specifically with the surface proteins of hepatitis B virus, these alternative helper viruses would likely be impervious.
The hepatitis D antigen proteins may prove promising targets for future drug development as they are central to the life cycle of the virus. Human RNA polymerases —enzymes that help with genetic transcription— are another area of interest. Hepatitis D virus hijacks these host proteins to make copies of its genome. Interrupt this process and you interrupt replication. As always, the best way forward is rigorous research: the more we know about hepatitis D virus, the better equipped we are to neutralize it.

