Feast Your Eyes On DARPins For Vision Loss
(Posted on Wednesday, July 19, 2023)
Originally published on Forbes on 7/14/2023

This story is part of a series on the current progression in Regenerative Medicine. This piece is part of a series dedicated to the eye and improvements in restoring vision.
In 1999, I defined regenerative medicine as the collection of interventions that restore to normal function tissues and organs that have been damaged by disease, injured by trauma, or worn by time. I include a full spectrum of chemical, gene, and protein-based medicines, cell-based therapies, and biomechanical interventions that achieve that goal.
Currently, the primary treatment option for wet age-related macular degeneration involves the injection of anti-vascular endothelial growth factor. These Anti-VEGF injections can be expensive, require frequent administration, and cause adverse effects such as vision problems, eye infections, and retinal detachment. This has driven research into alternative therapies for macular degeneration, one of which is DARPins.
Macular degeneration is an age-related eye disease that affects the macula, which is the part of the retina responsible for sharp, central vision. The disease causes a progressive loss of vision that starts in the center of the visual field and can advance to total blindness.
There are two types of macular degeneration: dry and wet. Dry macular degeneration is the most common form and is characterized by the slow breakdown of cells in the macula and the accumulation of debris in the retina. Wet macular degeneration, on the other hand, is less common but more severe, as it is characterized by the growth of abnormal blood vessels underneath the retina. These blood vessels can leak and cause permanent damage to the retina, leading to rapid vision loss.
Designed Ankyrin Repeat Proteins (DARPins) are synthetic proteins that show promise as a treatment. These engineered proteins exhibit powerful therapeutic potential as they can target the factors contributing to blood vessel growth and leakage, which are characteristic traits of macular degeneration.
With their innovative design, promising preclinical and clinical data, and potential to provide a new chapter in treating retinal disorders, DARPins hold great promise for improving outcomes for patients with wet age-related macular degeneration and related conditions.
What is a DARPin?
DARPins, a class of engineered proteins, hold immense promise and have garnered significant attention due to their versatility and potential biomedical applications. These proteins are derived from ankyrin repeat motifs, which naturally occur in protein domains across various organisms.
Cartoon representation of the molecular structure of protein registered with 2qyj code. EUROPEAN BIOINFORMATICS INSTITUTE
Ankyrin repeats consist of approximately 33 amino acids. These repeats intricately fold into specific shapes, forming highly stable protein domains. This inherent stability allows ankyrin repeats to play crucial roles in diverse cellular processes, contributing to their functional diversity.
DARPins are created by repeating and rearranging these units to achieve specific binding properties and target recognition. This modular design empowers DARPins to be customized and optimized for various applications, including therapeutic agents or diagnostic tools. The exceptional specificity and affinity of DARPins make them an ideal option for binding to complex molecules, such as disease-specific biomarkers and specific protein receptors.
Benefits and Drawbacks of DARPins
DARPins offer a multitude of advantages over antibody treatments for retinal disorders. Their compact structure allows better tissue penetration, including the retina, resulting in improved therapeutic delivery. Furthermore, DARPins can be engineered with exceptional affinity and specificity for target molecules, enhancing their therapeutic efficacy while minimizing off-target effects. They also exhibit remarkable stability, resisting degradation by proteases, making them a superior choice for retinal disorder treatment.
While assessing the advantages, we must also consider the potential drawbacks. It is crucial to recognize the intricacies involved in manufacturing, which demands specialized facilities and expertise. Additionally, ongoing clinical development, regulatory approval, and managing risks associated with immunogenicity and delivery challenges require further research and advances in protein engineering to fully unlock the potential of DARPins and overcome limitations associated with their utilization.
Clinical Trials of DARPins for Retinal Diseases
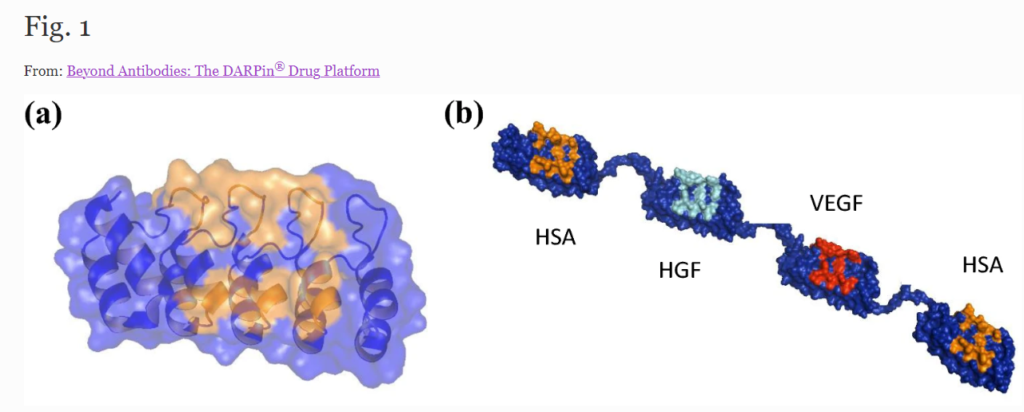
Representations of a single designed ankyrin repeat domain and a multidomain DARPin® drug candidate. Molecular model of MP0250, a DARPin® drug candidate consisting of four DARPin® domains (molecular weight approximately 62 kDa). The scaffold is dark blue, and the potential target interaction residues of the individual domains are orange (human serum albumin [HSA]), cyan (hepatocyte growth factor [HGF]), and red (vascular endothelial growth factor [VEGF])BEYOND ANTIBODIES: THE DARPIN® DRUG PLATFORM. BIODRUGS
Among the DARPins currently in clinical trials for retinal diseases, some of the most notable candidates include abicipar pegol, RG7716, and conbercept.
Abicipar pegol is a therapeutic agent targeting vascular endothelial growth factor-A (VEGF-A) in treating neovascular age-related macular degeneration and diabetic macular edema. Clinical investigations have reported that abicipar pegol demonstrated comparability to ranibizumab regarding efficacy, with a similar safety profile. However, there are some potential limitations to the therapy, such as an increased incidence of intraocular inflammation observed in some patients compared to other anti-VEGF therapies.
RG7716 is a bispecific DARPin binding to VEGF-A and Angiopoietin-2 (Ang-2). This unique feature holds promise in effectively blocking pathological angiogenesis, thus preventing blindness. Clinical trials have reported that RG7716 shows a statistically significant improvement in best-corrected visual acuity (BCVA) over the currently accepted therapy of aflibercept injections in patients with neovascular age-related macular degeneration. However, RG7716 has been associated with mild to moderate fever in some patients, and its use is not recommended for patients with a history of significant infusion reactions.
Conbercept is an innovative fusion protein that combines a DARPin domain, specifically designed to target VEGF receptors, with a domain derived from a human immunoglobulin. Clinical trials have shown its efficacy in treating various retinal disorders, including neovascular age-related macular degeneration and macular edema associated with retinal vein occlusion. Conbercept has also exhibited higher binding affinity to VEGF than other anti-VEGF agents, resulting in superior inhibition of pathological angiogenesis. However, a potential limitation of the therapy is that it is administered through intravitreal injection, which can cause adverse effects such as intraocular inflammation or endophthalmitis.
DARPins Pave the Way for the Future
DARPins, short for Designed Ankyrin Repeat Proteins, have emerged as a highly promising class of engineered proteins. Their versatility and wide range of applications show immense potential in treating various eye-related disorders. These proteins possess significant binding properties and exceptional stability, making them well-suited for biomedical purposes. While there are challenges in manufacturing and regulatory approval, ongoing research and continuous advancements in this field steadily drive their potential even further. The exploration of DARPins holds tremendous promise for the future of healthcare and for improving patients’ lives.

