Induced Pluripotent Stem Cells For Age-Related Macular Degeneration
(Posted on Sunday, August 27, 2023)
Originally published on Forbes on 8/22/2023

This story is part of a series on the current progression in Regenerative Medicine. This piece is part of a series dedicated to the eye and improvements in restoring vision.
In 1999, I defined regenerative medicine as the collection of interventions that restore to normal function tissues and organs that have been damaged by disease, injured by trauma, or worn by time. I include a full spectrum of chemical, gene, and protein-based medicines, cell-based therapies, and biomechanical interventions that achieve that goal.
Age-related macular degeneration is a progressive eye disorder that leads to severe vision loss in adults over 50. It is caused by damage to the macula, a small area of the retina responsible for sharp central vision, and retinal pigment epithelium cells responsible for nourishing the retina. Age-related macular degeneration affects the middle part of the vision, where detailed activities such as reading and driving are the most important. The disease has no cure, but novel interventions can slow or reverse its progression.
One such intervention is stem cell therapy. More specifically, induced pluripotent stem cells (iPSCs) show great promise as a treatment for age-related macular degeneration, as revealed by recent research published in the New England Journal of Medicine. The study involved transplanting autologous iPSC-derived retinal pigment epithelium (RPE) cells in a patient with the disease. The transplantation using a sheet-like structure was safe, well-tolerated, and improved visual acuity without leakage.
These findings and similar research provide strong evidence supporting induced pluripotent stem cells as a promising therapeutic option for individuals with macular degeneration. The versatility of these cells allows them to differentiate into any cell type, expanding their potential applications in the field.
What are Stem Cells?
Stem cells are undifferentiated cells that can specialize into any cell type in the human body. However, their ability to differentiate becomes limited as they become more specialized.
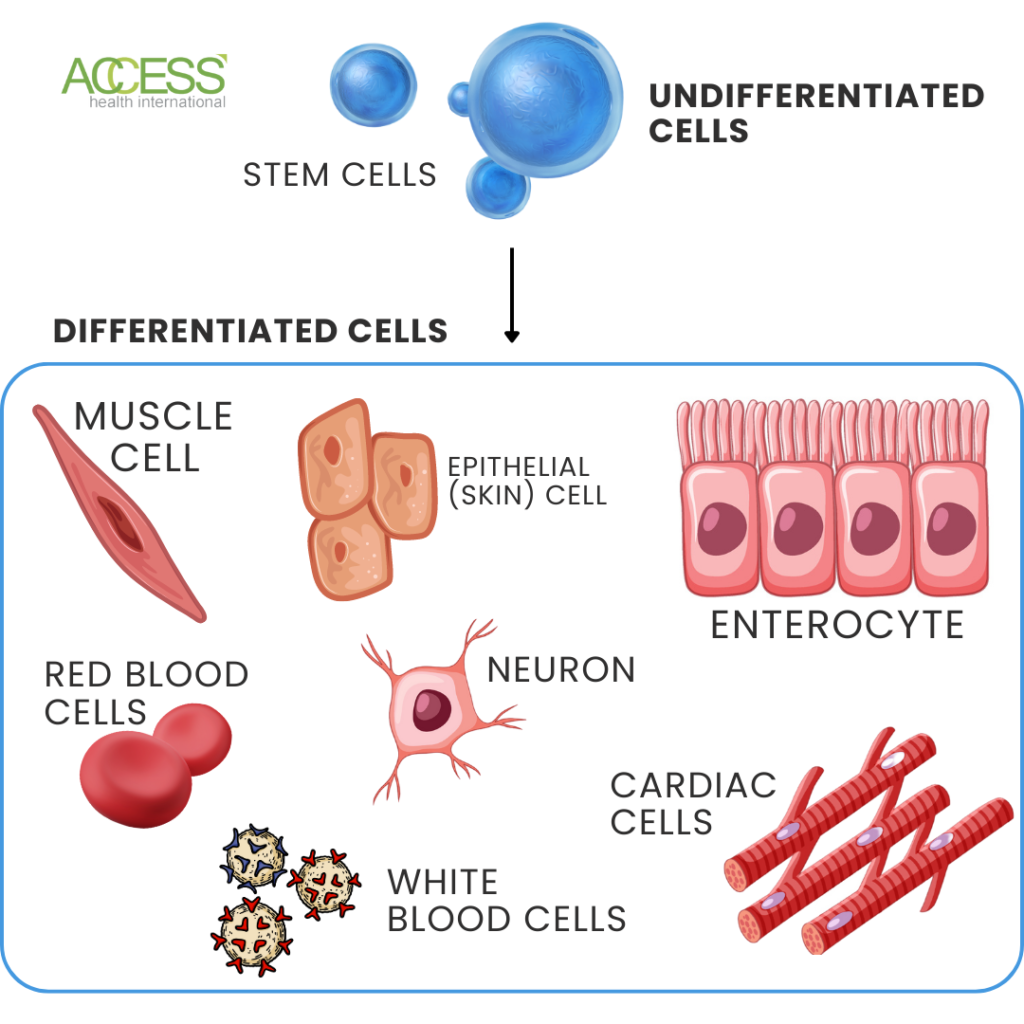
Stem cells also have the potential to repair tissue and restore function after injury or disease. Because of this potential, they can replace or repair damaged cells in the retina. But how do researchers know what type of stem cells to use?
Stem cells can be classified based on their ability to specialize. Totipotent stem cells can become any tissue in the body, pluripotent stem cells can become any cell type except for a complete organ, and multipotent stem cells can only differentiate into specific tissue types.
Using Induced Pluripotent Stem Cells for Macular Degeneration
Induced pluripotent stem cells (iPSCs) show promise in treating retinal degenerative diseases. They are created by reprogramming adult cells using Yamanaka factors, allowing them to revert to an embryonic state. These cells provide a virtually unlimited cell source for research and potential therapies.
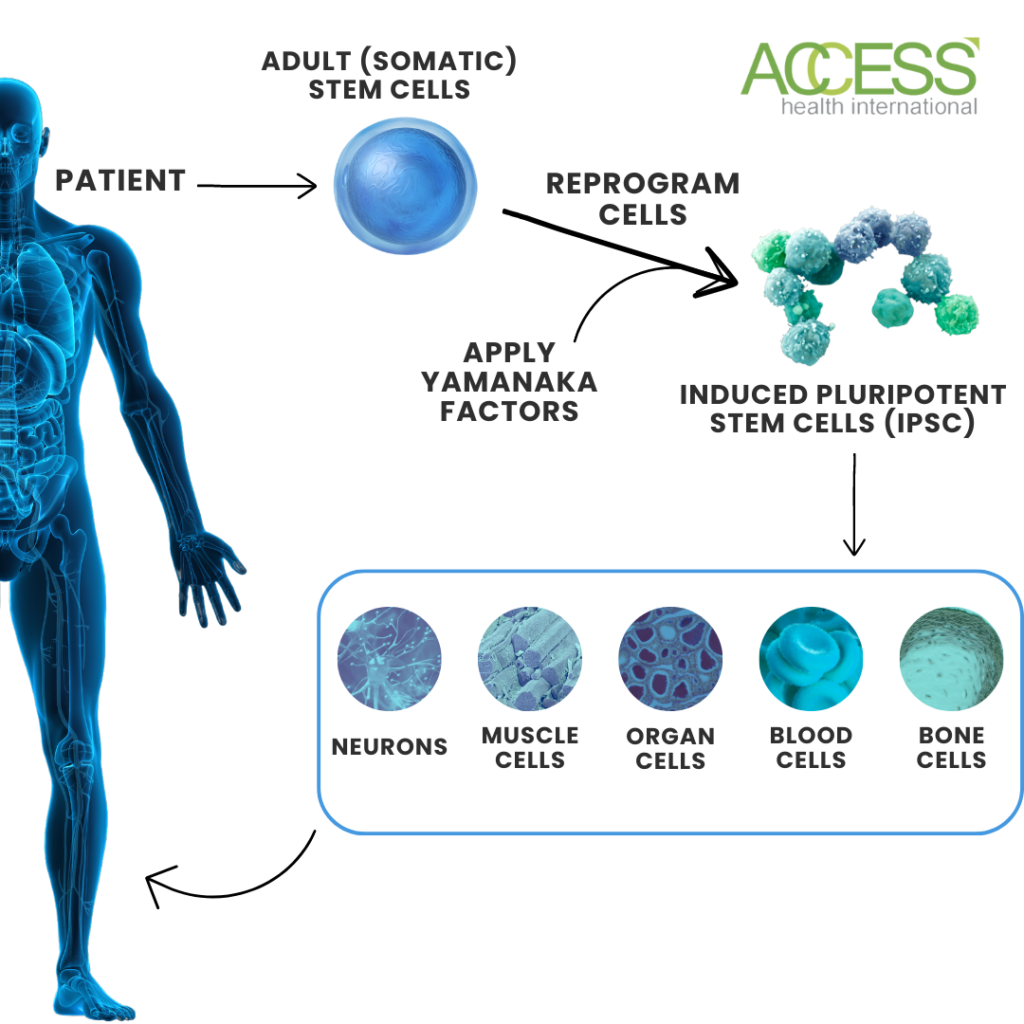
Scientists are researching several diseases and drug development applications for these cells, highlighting the characteristics that make them an ideal therapy for macular degeneration.
As macular degeneration advances, it can lead to damage in the retinal pigmented epithelium (RPE) and subsequent vision loss. However, there is a promising solution – developing iPSC-derived RPE transplants, as seen earlier. These transplants, created from induced pluripotent stem cells, have been developing for over a decade. They have shown great success without encountering significant systemic complications in multiple tests.
Researchers at the University of Pennsylvania are working to determine the best technique for restoring vision with iPSC therapy. One challenge is safely delivering a large number of cells without causing harm to the eye. They tackled this by injecting cells directly into the back of the retina, avoiding the removal of the vitreous or transparent gel layer of the eye. Immunosuppression will also be critical to prevent rejection. Additionally, integrating cells is essential, and injecting them into a retina with normal photoreceptor cells helps them to connect and send visual signals to the brain.
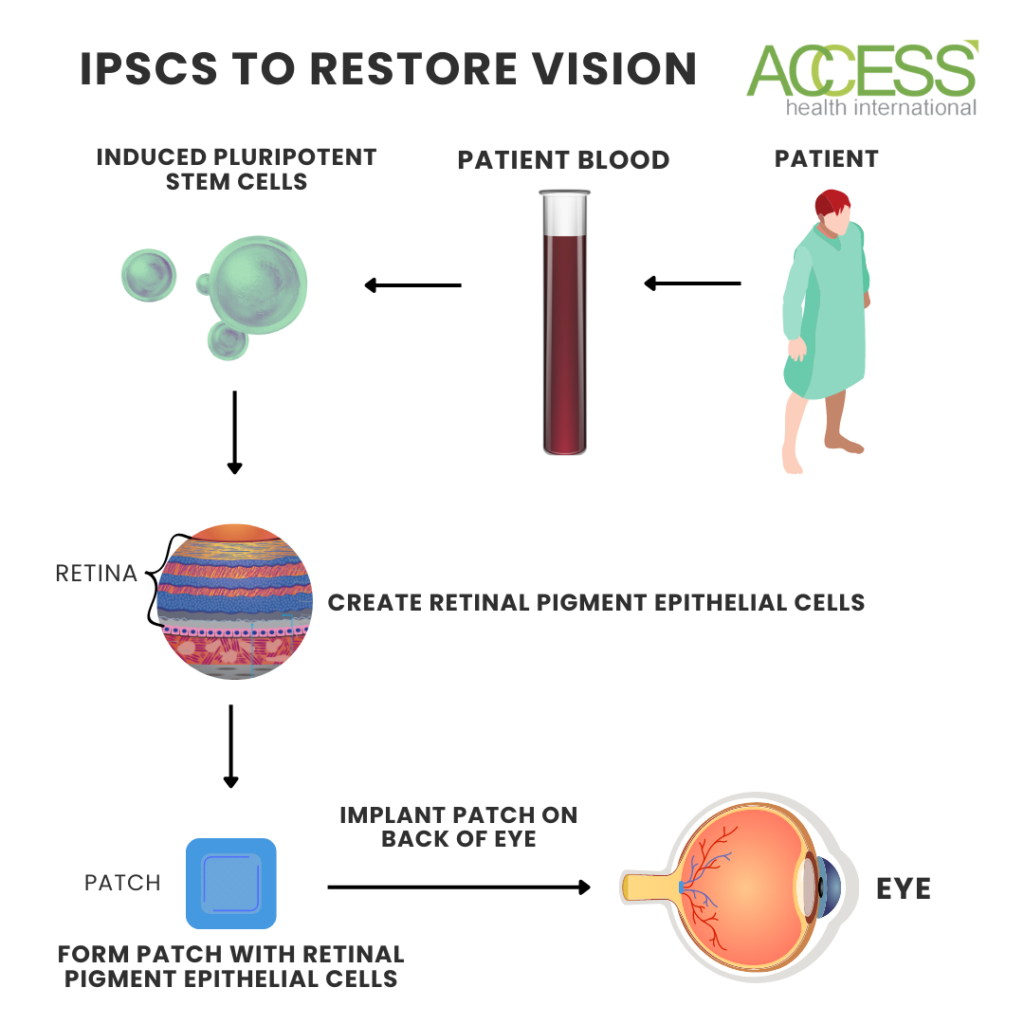
The New York Stem Cell Foundation, or NYSCF, is also developing a cell therapy to treat age-related macular degeneration using technology pioneered by the National Eye Institute and Columbia University. They aim to limit repeated treatment and cure blindness by using a patient’s cells to treat their degeneration. The team removes blood cells via a standard blood draw and uses advanced techniques to turn them into induced pluripotent stem cells, which takes roughly three months.
From here, they then turn the retinal precursor stem cells into retinal pigment epithelial cells. They do this using a recipe the National Eye Institute developed that directs the cells to transition. The cells then get transferred into a small patch that is smaller in size than a tic tac. That patch is then implanted into the back of the eye, where the cells integrate into optic neurons and restore vision. This process takes an additional two and a half months.
Future Outlooks
These groups are not the only ones investigating these unique cells to treat retinal degenerative diseases. Researchers in both London and California have launched Phase 1/2a clinical trials for stem cell therapy in treating age-related macular degeneration. In their phase 1 study, two patients with severe age-related macular degeneration had failed to respond to anti-VEGF treatments. The patients underwent a procedure where a retinal pigment epithelium patch was administered. This patch was meticulously placed in the subretinal space of one eye per patient using a specialized microsurgical tool. After placement, the cells from the patch underwent in vivo maturation, thus replacing the damaged cells with healthy ones. Both patients experienced a substantial enhancement in their best-corrected visual acuity within 12 months.
Similar to any clinical trial, the primary objective of this study is to establish both the effectiveness and safety of stem cells and induced pluripotent stem cell therapy for macular degeneration. While these treatments are relatively new, they are not without risks and potential side effects.
Immunosuppression and immune reactions pose inherent risks in cell-based therapies. It is necessary to deliberately suppress the immune system during cell-based treatments to prevent potential attacks on the administered cells. Interestingly, using iPSCs offers an alternative to this requirement as it allows for the extraction of cells directly from the patient, subsequently reintroducing them. This approach effectively circumvents the immune system’s response to foreign cells, eliminating potential red flags.
Alas, immunosuppression or immune response are not the only risks, and one potential risk that is more common to induced pluripotent stem cells is the risk of tumor formation. iPSCs are more likely to form teratomas than other types of cells used in cell therapies. Teratomas are tumors that contain cells from multiple germ layers and can be dangerous.
Aside from the biological challenges associated with this type of treatment, there is also a financial obstacle due to the time-consuming nature and cost associated with the cells. Recall that it takes 3-6 months to craft the cell patch for just one patient since the treatment is personalized.
Despite these obstacles, iPSC therapy has shown promise as a possible treatment option for macular degeneration, and ongoing and upcoming clinical trials will help us to see a future free from vision loss due to retinal degenerative diseases like age-related macular degeneration. What comes next will undoubtedly change the landscape of eye treatments and stem cell therapies.
To learn more about the eye, read more stories at www.williamhaseltine.com

