How mRNA Could Safely Replace Blood Stem Cell Transplantation
(Posted on Monday, August 28, 2023)
Originally published on Forbes on August 23, 2023.
This story is part of a series on the current progression in Regenerative Medicine. In 1999, I defined regenerative medicine as the collection of interventions that restore to normal function tissues and organs that have been damaged by disease, injured by trauma, or worn by time. I include a full spectrum of chemical, gene, and protein-based medicines, cell-based therapies, and biomechanical interventions that achieve that goal.
As part of a trio of stories on advances in stem cell gene therapy, this piece discusses how to alter blood stem cells using mRNA technology. Previous installments describe how the same platform could reinvent how we prepare patients for bone marrow transplants and correct pathogenic DNA.
At present, the only way to cure genetic blood disorders such as sickle cell anemia and thalassemia is to reset the immune system with a stem cell transplantation. Only a fraction of patients elects this procedure, as the process is fraught with significant risks, including toxicity and transplant rejection. A preclinical study published in Science explores a solution that may be less toxic yet equally effective: mRNA technology. The cell culture and mouse model experiments offer a compelling avenue for future research to enhance or replace current stem cell transplantations altogether.
The Risks of Transplantation
All blood cells in the body, healthy or diseased, originate from hematopoietic stem cells. These long-term stem cells continuously regenerate and produce blood cells throughout a person’s lifetime. Replacing diseased stem cells with healthy ones can cure people of their genetic blood condition by rebuilding the immune system.
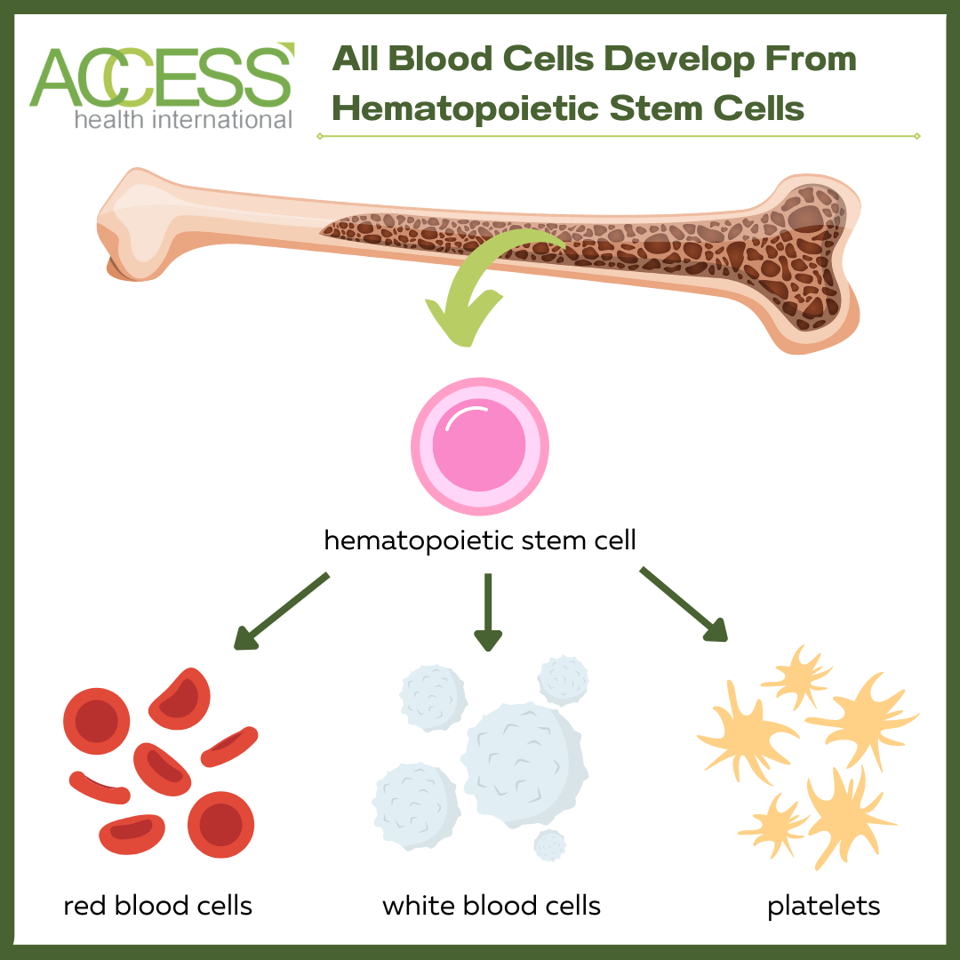
There are two methods to replenish a person’s stem cells, each with its own complications.
The first and most common method is to source healthy stem cells from a donor. Sourcing stem cells from others, or allogenically, is difficult due to the risk of transplant rejection. If the transplant is not well matched to the host, the body will view the new cells as foreign intruders and attack the graft. This phenomenon is known as graft-vs-host disease (GvHD). The associated risks decrease if a close relative, such as a brother or sister, offers to be a donor. However, only some patients have an eligible relative who can donate, and even then, rejection could still occur.
The other alternative is to use the patient’s stem cells instead. The diseased stem cells are corrected in the lab using gene addition or gene editing techniques before transplantation. While this solves donor-matching or tissue rejection issues, it is costly to extract and gene edit a batch of stem cells for each patient. The expense of specialized, small-scale manufacturing limits this option’s accessibility—a problem faced by other personalized gene therapies (e.g., CAR T therapy).
Lastly, it is difficult to avoid the toxic risks of chemotherapy or radiation, which may be used to prepare the patient’s bone marrow for either type of transplantation.
A Novel Solution: Editing via mRNA Technology
Researchers at the University of Pennsylvania explored the possibility of mRNA technology to enhance how we treat genetic blood diseases. The technology relies on antibody-covered lipid nanoparticles to deliver genetic information (mRNA) to a target cell; the antibodies establish the cell target. Upon arrival, the nanoparticle is encircled by the target cell and slowly broken down once inside the cell. Eventually, the genetic material is released into the cell cytoplasm, where cell machinery can read it to initiate immune responses. The hope is to deliver gene-editing instructions to a patient’s stem cells via a single mRNA injection, circumventing the need to extract and edit the cells in a specialized lab.
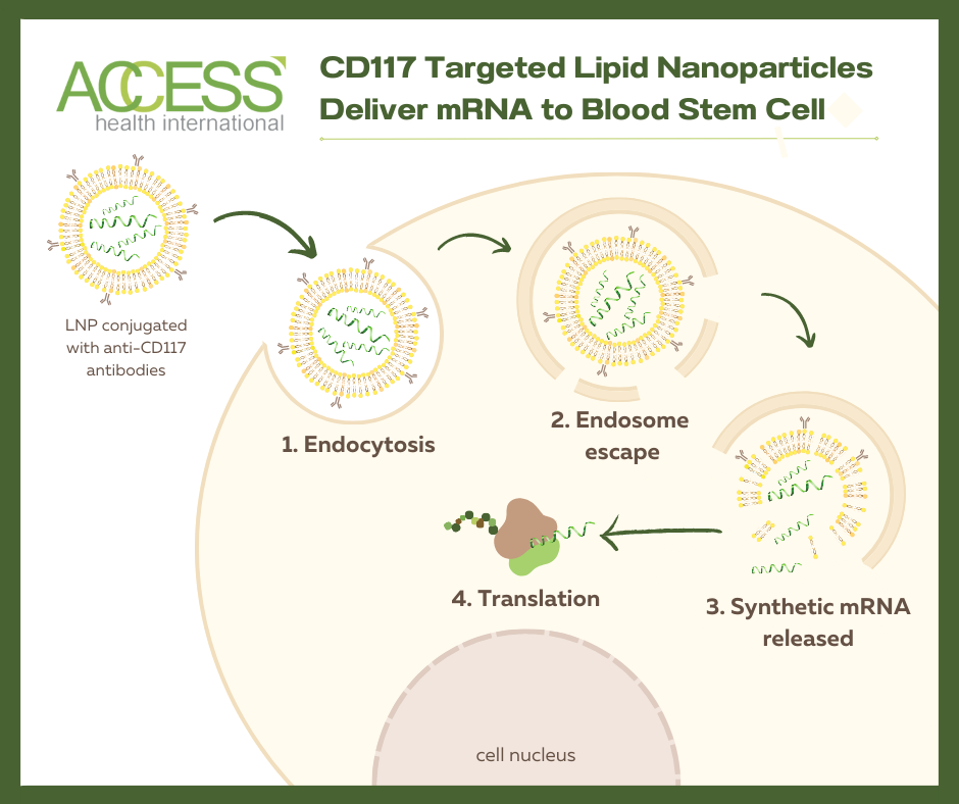
mRNA-Edited Stem Cell Transplant
Could antibody-covered lipid nanoparticles successfully edit hematopoietic stem cells? To test this, lipid nanoparticles decorated with anti-CD117 antibodies were used to treat a batch of bone marrow cells. These antibodies effectively detect CD117 antigen on hematopoietic stem cells. Inside the nanoparticles lay modified mRNA called Cre, short for cyclic AMP response element. If the mRNA is successfully delivered, the reporter gene inside the bone marrow cells should activate in response to the Cre-mRNA, turning the cell culture from red to green fluorescent. The researchers can then assess for gene editing through this color change.
Mice received either the edited bone marrow cells or bone marrow cells treated with a control intervention. The team noted reporter gene expression for four months. The results demonstrate near-complete gene editing for mice treated with CD117-decorated, Cre-carrying lipid nanoparticles. Red blood cells, white blood cells and other immune cells show evident reporter gene expression, while long-term blood stem cells exhibit high levels of gene editing. The editing rates appear dose-dependent, with higher doses resulting in higher editing percentages. When these bone marrow cells are transplanted again into another group of mice, the stem cells maintain their ability to generate different types of blood cells.
In Vivo Editing in Mice
The ultimate goal is to gene-edit blood stem cells in vivo using a single injection of mRNA-carrying lipid nanoparticles. To mimic this, the investigators attempted to alter the hematopoietic stem cells inside the mice instead of editing the bone marrow cells first.
Mice received an injection of either CD117 lipid nanoparticles with Cre mRNA or control lipid nanoparticles. Reporter gene expression was monitored for four months. Impressively, the intervention mice experience significantly higher editing in peripheral blood cells and long-term blood stem cells than control mice. Editing levels increased with larger doses of intervention treatment. The green color change persists even when bone marrow cells from the first set of mice are transplanted into a secondary set of mice, validating the successful editing of long-term blood stem cells.
Targeting Other Tissues?
The CD117 lipid nanoparticles demonstrate spleen and bone marrow uptake—a positive sign of accurate targeting and mRNA delivery. This is because blood stem cells largely rest in the bone marrow; in turn, the spleen supports the maturation of blood cells derived from these stem cells.
The team also checked to see if other tissues were affected by the lipid nanoparticles. They find reporter gene expression in the liver for both the control and intervention nanoparticles. The result points to a known phenomenon in which the liver interacts with receptors on the lipid nanoparticles. In the lung, the intervention nanoparticles elicited significantly higher reporter gene expression levels than controls. This may be attributed in part to the lung cells carrying CD117, the desired antigen target for the nanoparticles.
The treatment does not impact the testis, according to this study. Cells from the testis in intervention mice do not stray from baseline. Moreover, the children from intervention and control male mice do not express the reporter gene.
Noting tissue uptake is essential for gauging off-target effects. The lipid nanoparticle system used here may need further refinement to prevent gene editing in unwanted organs.
Looking Ahead
mRNA technology possesses an expansive potential to improve gene therapy. The study authors here demonstrate how the platform can successfully target and edit stem cells in cell culture and inside mice. The researchers also show how the same platform could therapeutically correct the human genome and eliminate preparatory chemotherapy. Building upon these findings could lead us to a simple treatment that replaces current stem cell transplantation procedures with a single injection.
To read more of this series, please visit www.williamhaseltine.com

