Covid-19: The Shapeshifting Protean Virus
(Posted on Thursday, August 24, 2023)

https://www.metmuseum.org/art/collection/search/397434
Originally published on Forbes on 8/24/2023.
SARS-CoV-2 is a protean virus. It seems designed to reinfect a previously infected host, changing its outer coat as well as some of its properties. The virus is also adapting to a number of new environments, including human and other animals. As a result, we must apprehend the protean nature of the changes it undergoes and adapt our protective strategies, including vaccines, to this reality. This is not the first time humanity has encountered a virus that constantly changes, as we are all familiar with influenza.
The current SARS-CoV-2 concern is a new variant, BA.2.86, that appeared first in Israel, then Denmark, and most recently the United States. We all remember Omicron and how it swept the world that had already experienced several waves of Covid cases. There were so many changes in the outer spike protein of Omicron that previous infections did little to protect from new infections. How much previous infection protects from disease still remains in question.
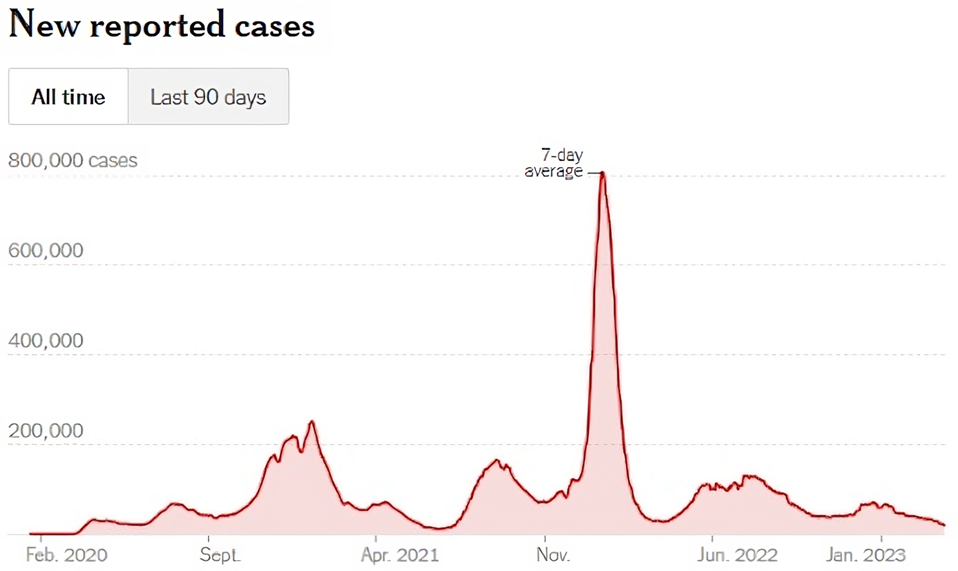
FIGURE 1: Omicron spike in Covid cases in the United States in early 2022.
The strain that led the Omicron spike in early 2022 was BA.2, which contained 54 amino acid mutations from the original Wuhan virus. The cause for concern with BA.2.86 is that it contains 41 amino acid mutations on top of the BA.2 mutations, totaling 95 mutations from the Wuhan virus. The concern is that BA.2.86 may or may not be the latest Omicron threat. Rather than wait to see if the virus spread globally, here I analyze this virus to summarize what it is and what is known about its properties.
BA.2.86 is likely a common descendant of one of the original Omicron variants, BA.2, and a more recent variant, XBB. In the spike protein of BA.2.86, we see 60 amino acid mutations, including substitutions and deletions. For context, the Alpha variant, which fueled the second-largest surge of cases in the United States behind the initial Omicron surge, contained just ten spike amino acid mutations.
We have seen many of the spike mutations in previous variants of concern, namely K417N, N440K, S477N, N501Y, and so on. However, over a dozen mutations are unique or rare in terms of previous variants. The figures below demonstrates the significant departure from the dominant XBB.1.5 variant, as well as a comparison to BA.2 and EG.5.1.
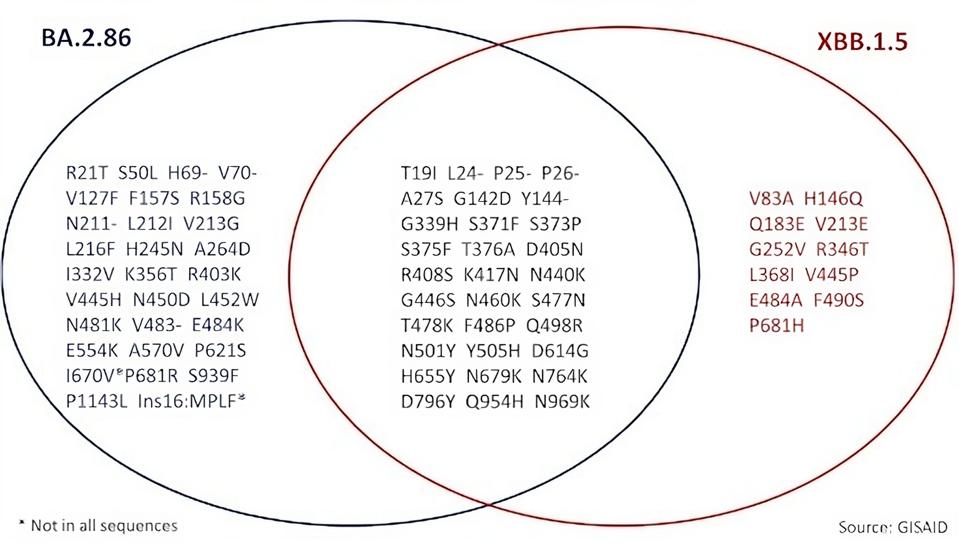
FIGURE 2: Spike mutational profile of newly designated BA.2.86 as compared to XBB.1.5
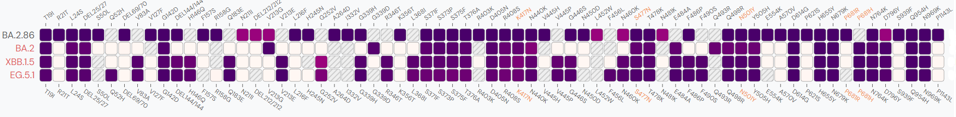
FIGURE 3: Spike comparison between BA.2.86 and BA.2, XBB.1.5, and EG.5.1.
Notably, the updated Covid vaccine set to be released this fall is designed to protect against the XBB.1.5 variant, but not BA.2.86. The hope is that the vaccine will protect against BA.2.86 should it widely circulate, but it would be unsurprising if the variant evaded booster protection, given the degree to which BA.2.86 is mutated in the spike is extreme.
Because changes in antigenicity are mostly, but not entirely related to the spike protein, I will first speak to spike mutations in greater detail. Each of the more than two dozen mutations found in BA.2.86, but not the dominant XBB.1.5, may give the new variant a distinct advantage, but I will discuss a few I find most notable.
The K356T mutation has been previously described as a mutation that could provide a significant immune evasion advantage to SARS-CoV-2. The mutation adds an N-glycosylation site to the receptor-binding domain at N354, which allows for more efficient antibody blockage. Further, patients treated with sotrovimab were found to have developed this mutation in their Omicron infections in response, again speaking to the immune evasion and resistance of K356T.
The numerous N-terminal domain mutations could prompt several similar N-glycosylation sites and are worth further investigation.
In the receptor-binding domain, there are several more mutations, all of which could work to improve ACE2 binding affinity or reduce antibody binding efficiency.
The P1143L mutation has been shown to potentially increase viral entry efficiency, which may be attributable to the mutated amino acid’s increased stability to the spike protein structure.
I want to draw your attention to mutations outside the spike region, which may be important for the pathogenicity and the spread of the virus. Throughout the genome, there are a wide variety of mutations in the Orf1ab replication-transcription complex (NSP1-16), some in the structural proteins (E, M, and N), and a few in the accessory proteins (Orf3a-8). The reason we bring attention to these is that mutations in some of these proteins, particularly the N protein, can make a significant difference in the replication of the virus.
Below is the entire catalog of mutations found throughout the virus.
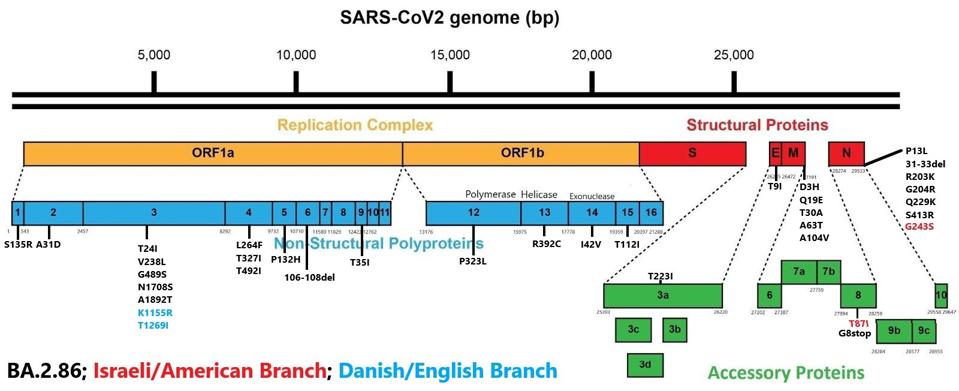
FIGURE 4: BA.2.86 nonspike mutations. Those in red are additional mutations found in the Israeli and American samples, and those in blue are additional mutations found in the Danish and English samples, as detailed in figure 2.
One significant protein of note that is heavily mutated in BA.2.86 is the NSP3 protein. There are as many as seven mutations in NSP3, namely T24I, V238L, G489S, N1708S, A1892T, and in some instances, K1155R and T1269I. NSP3 is one of the most active proteins in the virus, playing roles in viral RNA binding, polyprotein processing, and other functions. While the exact function of these mutations is unknown, they are likely to increase efficiency of many of these mechanisms, creating a more functional and pathogenetic virus.
Notably, branching is already occurring in the seven cases of BA.2.86. When looking at just the spike mutations, one may assume they are the same virus, but upon further inspection, we find that two of the cases—the Israeli and one of the Americans—contain three mutations not found in the others, namely N G243S, Orf8 T87I, and S I670V.
Four more cases—three from Denmark and one from England—have a difference of their own: NSP3 K1155R. The English case has an additional NSP3 T1269I mutation. The most recently detected case—an American traveling from Japan—contains none of these five listed mutations, creating its own branch.
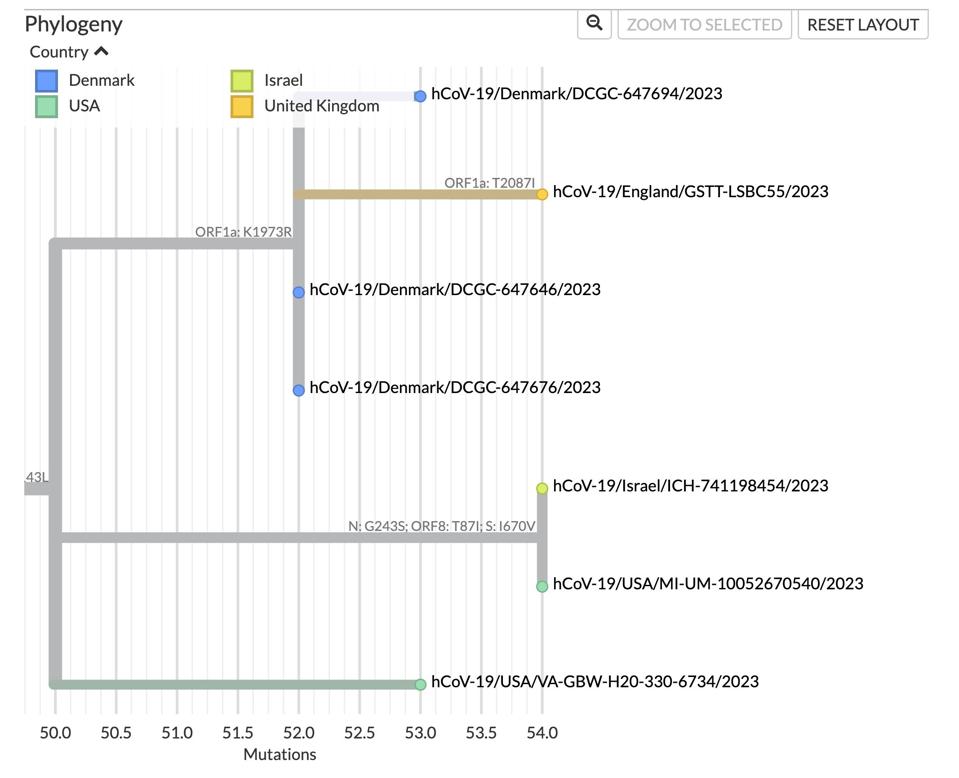
FIGURE 5: Differing lineages with the BA.2.86 framework.
One final note on mutations I must make is synonymous mutations, or those that do not result in an amino acid change. There are likely dozens of synonymous mutations littered throughout the BA.2.86 genomes. However, collecting data on these mutations is much more complex than amino acid mutations.
For instance, the termini of the virus, known as the five prime and three prime ends, often contain these synonymous mutations, as amino acid coding does not begin until NSP1 of the Orf1a complex. One of these synonymous mutations is C241T. Along with NSP12 P323L and S D614G, C241T was part of the first set of mutations to the original Wuhan wild type and continues to feature in every known variant of SARS-CoV-2.
While synonymous mutations do not impact the amino acid sequence of the virus, it does affect the tertiary structure of the virus’s RNA, which studies suggest can play a role in the adaptation of the virus to the human host environment.
For instance, a recent study on the SARS-CoV-2 N protein shows that the N-terminal domain of N recognizes and binds RNA sequences in the five prime untranslated region of the virus. The N protein is involved in RNA transcription and genome packaging into virus particles, a crucial role in virus transmission. Thus, altering the structures of the five prime end could impact the function and efficiency of N, impacting the overall viral function of SARS-CoV-2.
On whether BA.2.86 will cause a new wave of Covid cases akin to Alpha or Omicron in years past, I cannot say. With so few cases of BA.2.86 to date and so little data on its pathogenicity, many questions remain.
First and foremost, is this virus transmissible and disease-causing? We know that most of the patients were not traveling to a significant degree, but whether these patients are immunosuppressed, elderly, undergoing chemotherapy, or a similar treatment, we do not yet know. These populations are much more likely to develop severe disease than the average healthy adult, and the release of such information could be helpful in our health policymaking regarding BA.2.86.
Another pressing issue is that of the vaccine. Were the BA.2.86 variant to take hold and create a new wave of cases, would the latest vaccines be protective? Such data is, as of yet, unavailable. While these early detections of BA.2.86 are a preliminary shot across the bow, it remains to be seen how they will develop in the coming weeks and months.

