Walking The Path To Recovery: The Future Of Parkinson's Care
(Posted on Friday, December 22, 2023)

This article was originally published on Forbes on 12/22/23.
This story is part of a series on the current progression in Regenerative Medicine. This piece discusses advances in brain-machine interfaces.
In 1999, I defined regenerative medicine as the collection of interventions that restore to normal function tissues and organs that have been damaged by disease, injured by trauma, or worn by time. I include a full spectrum of chemical, gene, and protein-based medicines, cell-based therapies, and biomechanical interventions that achieve that goal.
There are between 500,000 and one million Americans suffering from Parkinson’s disease. The illness causes the neurons in their brain to break down and die gradually, resulting in tremors, neural processing issues such as memory loss or worsened concentration, and difficulty walking, among other symptoms.
Dr. Tomislav Milekovic and colleagues from the NeuroX Institute in Geneva developed a spinal cord neuroprosthetic to combat these symptoms, as described in Nature. The system reactivates nerves in the spine’s base associated with walking, rejuvenating the fading connection resulting from Parkinson’s.
Here, I will examine their neuroprosthetic and discuss the system’s impact on the future of Parkinson’s treatment.
I have analyzed and discussed many innovations in the brain-machine interface landscape for several months. It is worth noting that Milekovic’s neuroprosthetic is a slight departure from the rest. While brain-machine interfaces connect that brain or nervous system with a computer, neuroprosthetics connect the nervous system to a less advanced device, typically a motor or some other cognitive modality. Both achieve the same goal of restoring some intended cognitive function but through differing avenues.
Milekovic and colleagues focused their efforts on the restoration of walking movement in Parkinson’s patients. The disease impacts locomotion in several ways, but among the most important is gait abnormality. The stiffness and slowness of muscle movement result in small and inconsistent step sizes, impacting the ability to retain balance and turn.
Gait, speed, and balance are all controlled by the motor cortex at the crown of the brain, which is one of the more impacted neural sites for a Parkinson’s patient. The electrical signals are still created, but their movement down the spinal column worsens as the disease advances.
The neuroprosthetic’s design includes a pair of electrodes in the brain to monitor electrical signals and a second pair in the lower lumbar portion of the spine.
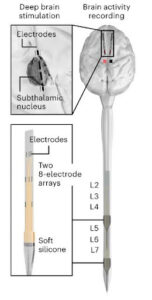
FIGURE 1: Scheme illustrating the insertion of DBS electrodes in the subthalamic nucleus of M9 and the anatomical location of DBS implants
The signals from the brain are relayed to an external processing unit that analyzes the incoming impulses and relays them to the electrodes in the lower lumbar, essentially creating an alternate path for the signal to reach the legs.
In non-human primates with similar motion impairments to a human with Parkinson’s, the neuroprosthetic “ immediately alleviated gait impairments and balance problems” in all three subjects. The system also improved posture and more difficult movements, such as climbing a ladder.
Milekovic and colleagues also extended their testing to one human participant. The 62-year-old man had suffered from Parkinson’s disease for 30 years, including extensive locomotive impairment.
As with the nonhuman primates, the neuroprosthetic alleviated gait impairment, improved balance, and reduced the frequency of freezing. Further, the human patient could wear external sensors and hardware that made the system more accurate and effective than in the nonhuman primates.
While these results are promising, there are some notable drawbacks to the study and the system. First and foremost, as the researchers emphasize, there needs to be more significant and larger studies on the system before widespread adoption. A one-person study, while indicative of potential, is not statistically significant.
Second, we do not know how neuroprosthesis affects Parkinson’s patients in the long term. It seems reasonable that continued invasive electrodes in the brain could impact neural degeneration over time.
Third, the system requires extensive fine-tuning to achieve perfect gait rehabilitation. This is not a matter of inserting electrodes and regaining walking ability. Rather, the system takes hours and hours of active use to learn the electrical signals involved in a patient’s walking, which varies from person to person.
This is not to say that the neuroprosthetic is not an achievement. In fact, this system can potentially improve thousands of people’s lives, but only with extensive research and development over the coming years.

