Next-Gen Brain Implants: Overcoming Challenges For A Seamless Connection
(Posted on Friday, December 29, 2023)
This article was originally published on Forbes on 12/29/23.
This story is part of a series on the current progression in Regenerative Medicine. This piece discusses advances in brain-machine interfaces.
In 1999, I defined regenerative medicine as the collection of interventions that restore to normal function tissues and organs that have been damaged by disease, injured by trauma, or worn by time. I include a full spectrum of chemical, gene, and protein-based medicines, cell-based therapies, and biomechanical interventions that achieve that goal.
The brain-machine interface is among the most exciting emerging fields in regenerative medicine and perhaps the kindling for the next stage of human evolution. The development of brain chip-enabled systems that connect our minds to computers is rapidly advancing.
However, brain implants are still limited in many capacities, most prominently their shelf life. While some brain chips have been designed to last decades or even up to a lifetime, many stop working after only a few months. This is not a mechanistic failure but rather the brain’s response to what it views as an invasive mass.
A recent study in Advanced Science by Dr. Alexandre Trotier and colleagues from the University of Galway found that brain implants may induce reactive scar tissue in the brain that impacts implant function.
Here, I will discuss Trotier’s findings and their potential solutions to this major stumbling block in brain implant design.
The brain, like our other internal organs, consists mostly of water and is prone to motion throughout the day. Within the rigid skull, the brain’s folds are in constant friction. While this is no issue for the average human being, it prompts a question for those with brain implants. What happens when the soft, shifting brain is in friction with a hard, rigid implant made of metal or plastic?
As expected, the brain is both sensitive, in that the friction with the implant causes inflammation and damage, and adaptive, as the organ is quick to defend itself.
Trotier notes that the brain develops glial tissue around the implant’s base in response to neural implant friction. Glial cells are neural structural cells that make up roughly half of the volume of bodily neural tissue. These cells are the nervous system’s foundation, supporting the more critical cells, such as neurons. Amassing around the implant’s bass, the glial cells create a sort of scar tissue that reduces friction between the implant and the brain’s healthy cells. Imagine the calluses developed by a weightlifter, creating a layer between the weight and the skin underneath.

FIGURE 1: Glial encapsulation of intracortical microelectrode. (A,B) Shows two stages of glial activation where astrocytes and microglial cells are activated and incapsulate probe at the site of injury.
On a more technical level, the implant’s electrodes create a void in the immediate vicinity, which Trotier calls the peri-electrode void. The presence of this void increases fluid shear stress, which activates the previously described gliosis.
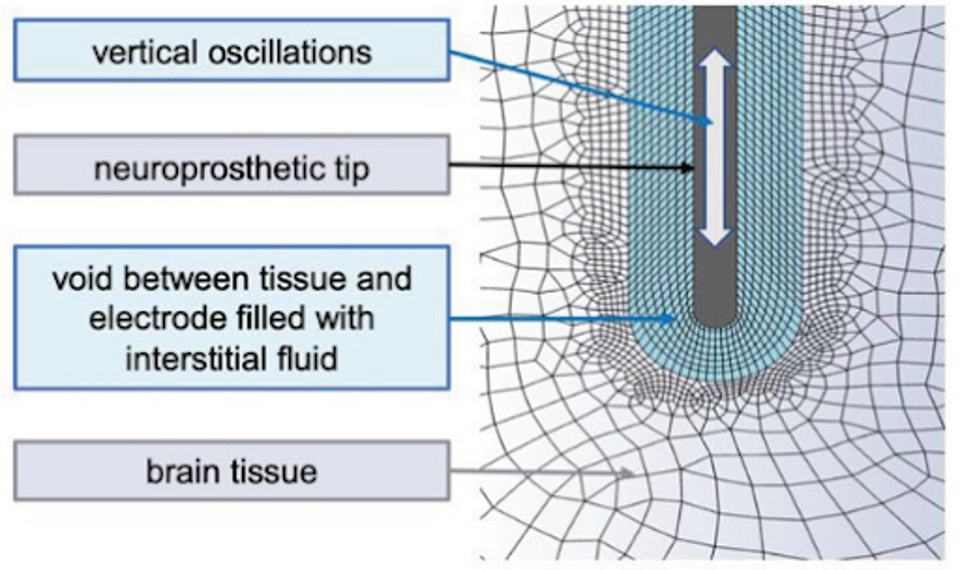
FIGURE 2: Schematic representation of peri-electrode void.
As you may suspect, significant additional tissue around the electrodes of a brain implant is likely to impact the functionality of the associated brain-machine interface. If the implant cannot read the signals the relevant neurons deliver, its presence in the brain is redundant.
Micromotion inflammation could seriously impede the future of brain-machine interfaces that rely on installed brain implants. How, then, can we reduce gliosis from taking place? One can either address micromotion itself or the inflammation and damage that occurs as a result.
One option for the former is enhanced osseointegration, or the structural connection between bone and implant. In terms of a neural implant, this may transpire as minimizing implant architecture within the skull, keeping as much of the system on the outside of the skull as possible, and reducing friction with brain matter. Another option may be a probe situated to the inner skull instead of the brain. This would require clever design and optimization but could make systems less prone to failure.
Another way to avoid micromotion is to shift focus to nonimplantable probes altogether. While not as accurate as implanted probes, recent advances in brain wave technology still yield comparable results regarding brain activation registration. Patients in these studies used a removable cap, as opposed to invasive brain probes.
If micromotion is unavoidable, Trotier and colleagues suggest a soft gel coating for the implant, acting as a barrier between it and the brain cells, preempting gliosis. A soft gel could reduce friction and lengthen implant lifespan.
Another option is to investigate electrode materials themselves. For instance, Dr. Joseph Pancrazio and colleagues are engineering probes using liquid crystalline materials with shape-changing capacities, potentially reducing the risk of significant scarring build-up.
Ultimately, these systems have significant research and development before being widely adopted and affordable in public healthcare. While the shortcomings of current brain implants affect those who use them now, these kinks will likely be ironed out in the next several years as such technology becomes a more mainstream regenerative treatment.

