Viral Awakening: The Hidden Threat of Human Herpes 6 (HHV-6) in CAR T Therapy
(Posted on Wednesday, December 13, 2023)

Before any treatment, each clinician and patient must determine whether the anticipated benefits outweigh the potential toll caused on the body. Study results published in Nature suggest that latent virus reactivation may be a valid point to consider for CAR T and other immunotherapies.
GETTY
All medicine—from Tylenol to the latest innovations in cancer care—is a balance of risk and reward. Each person must ask if the anticipated benefits outweigh the potential damage to the body. This is no different for patients who undergo CAR T therapy, a novel cancer immunotherapy that has yielded promising results for certain types of lymphomas, leukemias and multiple myeloma. Among already known risks such as cytokine storm and neurotoxicity, it is also possible to stir up viral infection, according to new research.
A study published in Nature points to latent virus reactivation as an understudied but important complication of CAR T therapy to consider. The authors discover the mechanism for why, in rare cases, a previously sleeping strain of herpes virus (HHV-6) can awaken during the CAR T process.
Latent Virus Reactivation: Waking the Beast
Humans are exposed to a wide range of pathogens when young. While the symptoms of the initial infection may come and go, some viruses remain in the body for life, hiding their genetic information in host cells while waiting to strike. If the person’s immune system is acutely weakened or stressed, the dormant pathogen eagerly reactivates its viral replication cycle and triggers a wave of new symptoms or illness. This phenomenon—the revival of a virus that has entered an inactive state within a host’s cells—is called latent virus reactivation.
Immunosuppression is a major trigger for these opportunistic viruses. The virus can take advantage of the imbalance between the virus and host, as there are fewer white blood cells to counter the attack. HIV/AIDS patients and organ transplant patients are particularly susceptible. In contrast, healthy people may not experience symptoms at all.
Human herpesviruses are common reactivation culprits. This family of nine viruses is known to establish latent infections in humans. Most people are exposed to at least one of these viruses by adulthood, but the clinical presentation of each pathogen differs. Table 1 lists the differences between primary infection and reactivation symptoms for each virus.
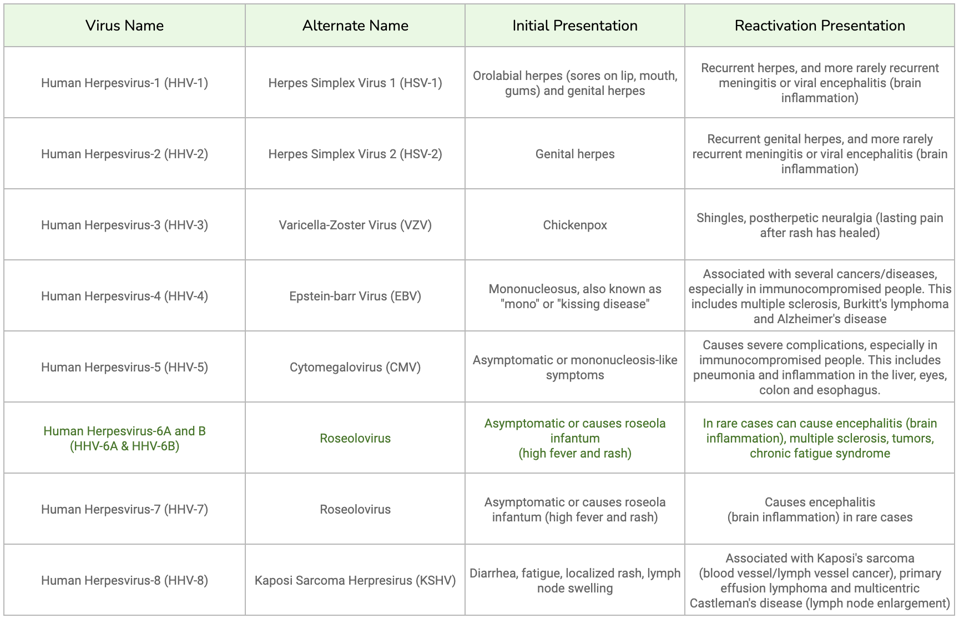
TABLE 1: Chart of all human herpesviruses, along with their initial presentation and reactivation presentation. Reactivation can be life-threatening for immunocompromised patients in particular.
ACCESS HEALTH INTERNATIONAL
Human Herpes Virus-6, or HHV-6, is actually a collective name for two distinct viruses: HHV-6A and HHV-6B. Both viruses replicate in T cells, but more is known about HHV-6B than HHV-6A. Over 90% of the human population is infected by HHV-6B by the age of three. The virus spreads through person-to-person contact, especially in daycare centers. Initial infection leads to rosela infatum, a childhood illness characterized by high fever and a mild rash, while reactivation has been linked to various complications including encephalitis (brain inflammation).
Human Herpes Virus-6 and CAR T Therapy
Although herpes reactivation is well-documented in immunocompromised patients, the latest cell therapies have yet to accumulate such data due to a lack of routine surveillance. These treatments rely on chemotherapy drugs to wipe out existing immune cells in the body. While the process prepares the patient for their cell infusion, it simultaneously weakens the body and leaves the patient susceptible to viral reactivation.
This is true for This is true for Chimeric Antigen Receptor T cell (CAR T) therapy, which the FDA approved in 2017 for treating resistant/refractory blood cancers. Some reports suggest that CAR T therapy can spark cytomegalovirus and HHV-6 reactivation and may cause subsequent neurotoxicity, but the specifics remain unknown.
HHV-6 Present in T Cells
Stanford University researchers recently chipped away at this mystery. In their paper, they relied on large-scale genomic analyses of viruses and single-cell RNA sequencing to understand why HHV-6 reactivates in a minority of CAR T cell patients.
Combing through Serratus, a cloud resource of all publicly available viral sequences, the team realized that HHV-6 RNA is expressed more than any other viral RNA in T cells. Then, they isolated white blood cells from healthy donors to mimic the CAR T cell process. The therapy usually entails extracting T cells from a patient; the cells are altered and proliferated in a lab to improve their cancer-fighting abilities; as previously mentioned, patients then undergo a preparatory course of chemotherapy before receiving their infusion of modified T cells. The experiment revealed that HHV-6 expression can increase in some T cells. A combination of various cues in the cells and during the manufacturing process likely upregulates a T cell receptor (OX40) the herpes virus uses to enter the cell.
Next came a cell culture analysis, a comparison of viral and human gene expression in the T cell populations of three healthy donors. The results showed that 0.1-0.3% of all the cells in culture reactivated or expressed HHV-6 at high levels. These HHV-6 “super-expresser” cells are mainly confined to CD4+ “helper” T cells, a subset of T white blood cells. Two of the donor samples were tested again several days later; on Day 25 or Day 27, the percentage of super-expressors increased to 49% and 62% of all T cells, demonstrating how a tiny collection of super-expressors can spread to other T cells in the population (including CD8+ “killer” T cells, another T cell subset).
CAR T Cells Reactivate HHV-6
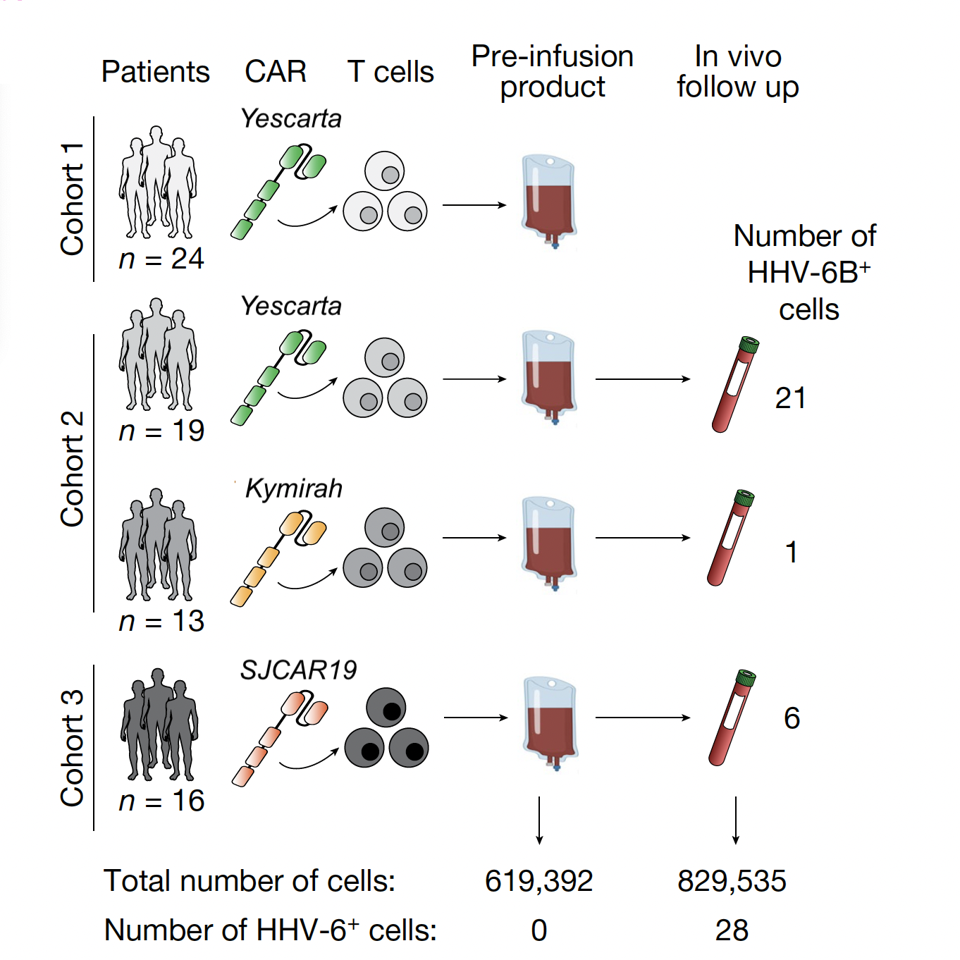
The team sought to understand how HHV-6 activation occurs in patients instead of cell cultures. Samples were taken from patients who underwent either FDA-approved or clinical trial CAR T products. All 76 samples were screened before CAR T cell infusion and after. Although none of the cells expressed HHV-6 prior to infusion, 28 cells expressed HHV-6 post-infusion. Additional testing suggested that HHV-6 can be detected between two and three weeks after the initial manufacture of the CAR T cell product.
Some experimental, ready-made CAR T therapies depend on T cells instead of the patient’s own T cells. Could HHV-6 be reactivated in these cases, too? The analysis of a single patient treated with ready-made CAR T cells reported HHV-6 expression on Days 14 and 19, suggesting that the longer duration needed to culture ready-made CAR T cells may increase HHV-6 expression.
The team also assessed if foscarnet, an intravenous medication used to treat certain herpesviruses, could lessen HHV-6 viral load. Donor-derived CAR T cells either received foscarnet or nothing on Day 24 of manufacturing. The results indicate a lower viral RNA abundance for foscarnet-treated cells than those with the untreated control.
FIGURE 1: A schematic of tested CAR T therapies, including FDA-approved (Yescarta, Kymirah) and experimental (SJCAR19) products. HHV-6 is undetected in all products prior to infusion.
LAREAU, C.A ET AL. (2023)
Takeaways
CAR T therapy can be transformative for many patients who qualify for it. Indeed, most patients will likely elect the therapy despite the risk of latent viral reactivation. However, gathering more knowledge on this understudied complication could minimize potential dangers.
HHV-6 reactivation in particular is poorly understood despite anecdotes of encephalitis and other toxicities in CAR T patients. By investigating the mechanism behind this reactivation, this paper lays a foundation of viral dynamics for current and future CAR T therapies to consider: both host cells and CAR T products can amplify pools of HHV-6 depending on the timing and conditions of each patient. As the authors test, antiviral medications may mitigate this rare threat; not mentioned is mRNA and lipid nanoparticle technology, a potentially superior alternative that turns T cells into CAR T cells inside the body instead of in the lab. This direct infection could forgo traditional lymphodepleting chemotherapy altogether.
This article was originally published on Forbes and can be read online here.