CAR T Therapy May Cause Rare Cancer & How CRISPR Could Be The Solution
(Posted on Sunday, December 24, 2023)

Digital illustration of genetic engineering.
GETTY
Could CAR T Therapy, an innovative treatment for certain blood cancers, cause lymphoma? Reports from clinical trials and data released after product approval suggest that the treatment could trigger the development of cancerous T cells in rare cases. Although the therapy’s benefits continue to outweigh this potential danger, the FDA recently announced an investigation on all six federally-approved CAR T therapies to understand this risk and evaluate the need for regulatory action.
Potential Culprits
Chimeric Antigen Receptor T cell therapy is a valuable therapeutic for patients with relapsed/refractory B-cell derived lymphomas, leukemias and multiple myeloma. The intervention works by using a patient’s own immune cells to treat their cancer.
White blood cells called T cells are extracted from the patient, genetically modified in the lab to equip synthetic receptors, and then multiplied to great numbers. The new, chimeric receptors enhance the T cell’s natural ability to identify and kill cancer cells. The patient receives an infusion packed with these CAR T cells after undergoing a short course of preparatory chemotherapy.
The gene modification process could be the culprit behind these recent reports. All FDA-approved CAR T therapies rely on inactivated viruses—retroviruses and lentiviruses, in particular—to deliver the chimeric receptor genes to the T cells. The viral DNA inside the viral is replaced with the CAR gene of interest and transfected into the T cell; then, the DNA permanently integrates into the target cell genome stably.
Viral vectors, while generally safe, carry the potential of insertional mutagenesis. This refers to viral DNA integrating into the host cell genome and accidentally activating cancer genes or inactivating tumor suppressor genes, and thus increasing the risk of cancer. This is not unprecedented, as two patients with severe combined immunodeficiency (SCID) developed leukemia after undergoing a gene therapy that relied on retroviral vectors. Similarly, one CAR T therapy study observed how introducing a CAR gene with a lentiviral vector disrupted a TET2 cancer gene in one patient with chronic lymphocytic leukemia; however, this disruption caused temporary CAR T cell proliferation and did not lead to secondary cancer.
The FDA acknowledged this intrinsic possibility upon approval of all CAR T products on the market and, in response, required a 15-year long-term follow-up for each therapy to monitor and assess the long-term safety profile.
The type of virus used could influence outcomes. Retroviruses generally have a higher risk of causing cancer because they tend to insert their genetic material randomly into the host genome, which can disrupt the normal functioning of genes. On the other hand, lentivirus vectors may not pose as much of a threat. Lentiviruses preferentially insert their genetic material into introns, non-coding regions of genes, that are actively being transcribed (turned into RNA). As introns are removed during the process of gene expression, lentiviruses are less likely to disrupt the normal functioning of genes, reducing the risk of causing cancer or insertional oncogenesis.
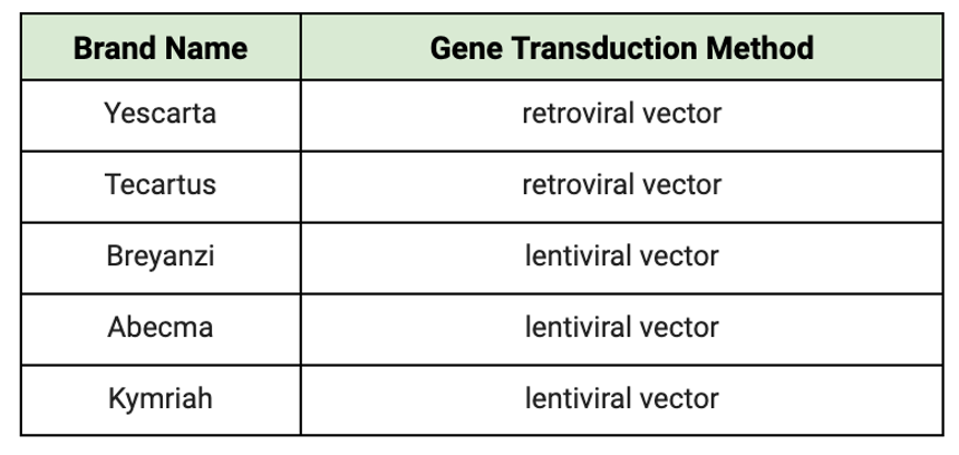
TABLE 1: Gene transduction method used for each CAR T cell product currently on the market. The current industry standard is to introduce the synthetic receptor genes to the T cell using viral vectors.
ACCESS HEALTH INTERNATIONAL
There is also the remote possibility that these patients already possessed a small population of cancerous T cells prior to undergoing treatment. The lymphodepletion chemotherapy required of all CAR T patients leaves the immune system weakened; already present cancer cells could take advantage of this vulnerable state.
CRISPR Gene Editing, a Possible Solution
If current gene editing methods place the patient at risk, it may be better to rely on other gene transduction methods. Notably, CRISPR-based editing could resolve many safety concerns associated with viral transduction.
CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) gene editing is a revolutionary system that can precisely edit DNA of one’s choosing. The system acts as a molecular scissor; it identifies certain DNA sequences and guides an enzyme to cut or modify the gene at a select location. With this, genetic material can be added, removed or altered at specific locations in the genome.
This novel method promises to be more accurate and efficient than viral transduction. Viral vectors rely on the virus’s ability to introduce genetic material to the cells, which can lead to random integration of genetic material into the host cell. Here is where off-target effects and unintended mutations can occur.
CRISPR is not yet integrated into any CAR T products in the market, but this paradigm may soon shift. The platform is often used in CAR T therapy research and gene therapy investigations in general. Just recently, the first CRISPR-based gene therapy was approved in the UK and the United States for patients with sickle-cell disease and thalassemia. While long-term monitoring will also be necessary for this system, preliminary results leave ample room for hope.
Looking Forward
While CAR T Therapy has demonstrated remarkable efficacy in treating certain blood cancers, the recent scrutiny regarding its potential association with T cell malignancies prompts a thorough examination of safety protocols. The inherent risks tied to the use of viral vectors in the gene delivery process may result in patients developing rare, secondary T cell cancer. Although not yet a standard in CAR T products, CRISPR’s precision and efficiency in gene editing present a potential avenue to mitigate concerns associated with this viral transduction. CAR T cells represent one arena in which CRISPR could open new doors for safer and more targeted therapies.
This article was originally published on Forbes and can be read online here.