How Regulatory T Cells Can Reduce Solid Tumors in Mice
(Posted on Monday, November 13, 2023)
This is the first installment in a group of stories on regulatory T cell function and its potential to treat cancer. This work falls within a greater series on regenerative medicine.
In 1999, I defined regenerative medicine as the collection of interventions that restore to normal function tissues and organs that have been damaged by disease, injured by trauma, or worn by time. I include a full spectrum of chemical, gene, and protein-based medicines, cell-based therapies, and biomechanical interventions that achieve that goal.
Research efforts in recent years often recognize and enhance the immune system’s natural ability to counter cancer, as exemplified through advances such as CAR T therapy and checkpoint inhibitors. In a similar vein, a study published in the journal PNAS discovered a method to unleash the immune system’s previously suppressed ability to fight solid tumors, a particularly difficult foe for recent immunotherapies to penetrate. Targeting the activity of a specific immune cell called regulatory T cells abolishes pre-established tumors in mice and demonstrates the potential of harnessing regulatory T cells to safely counter cancer.
What are Regulatory T Cells?
Regulatory T cells, also known as Tregs, are a small part of a larger family of white blood cells called the T cell lymphocytes. This subset of cells expresses two characteristic surface proteins—CD4+ and CD25+—alongside forkhead box P3 (FOX3), a transcription factor that helps the cell grow and function.
The purpose of these T cells is to maintain balance in the immune system. Just as a traffic officer manages vehicle flow to prevent accidents, regulatory T cells suppress other immune cells to minimize damage caused by excessive immune responses. The inhibition restrains the overactive immune system from mistakenly attacking the body’s own tissues, thereby limiting autoimmune and chronic inflammatory diseases—think type 1 diabetes, asthma or allergies.
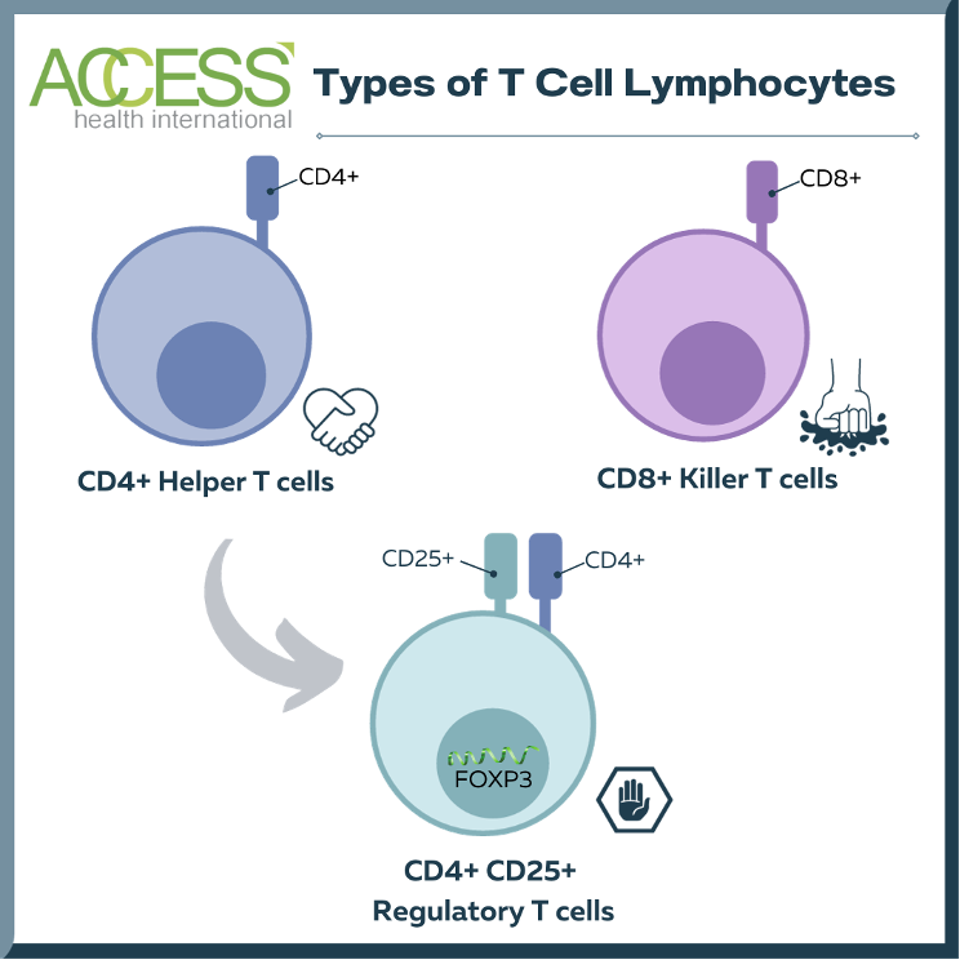
FIGURE 1: Comparison of different types of T cell lymphocytes. Most T cell lymphocytes are either helper T cells or killer T cells. Regulatory T cells are a subset of helper T cells that suppress other immune cells. While regulatory T cells and helper T cells both express surface protein CD4+, regulatory T cells characteristically possess an additional surface protein (CD25+) and transcription factor (forkhead box P3, FOXP3). This chart does not mention other T cells, including memory T cells, γδ T Cells, and natural killer T cells.
ACCESS HEALTH INTERNATIONAL
Suppressing Helpful Responses
While a necessary component to maintaining homeostasis, regulatory T cells also play a hand in tampering with otherwise helpful immune responses. This is the case with solid tumors. The tumor microenvironment (TME) actively recruits regulatory T cells to the site to hinder killer T cells and other immune cells from effectively combating cancer. New anticancer investigations explore strategies to control or reduce regulatory T cell activity within the tumor environment, thus empowering the immune system to overcome this suppression and mount a more robust response.
Potential Solution: Targeting Protein SRC-3
Researchers at Baylor College of Medicine are discovering ways to reduce Treg suppression and thus liberate the immune system to fight cancer. In two distinct stages, the team demonstrates how targeting a protein called steroid receptor coactivator 3 (SRC-3) in regulatory T cells could translate to effective antitumor activity.
Gene Knockout Safely Eliminates Tumor
Steroid receptor coactivator 3 (SRC-3, pronounced Sark Three) is a protein involved in regulatory T cell gene expression. As this protein is highly expressed in Treg cells and controls a wide range of genes, the researchers hypothesized that disrupting this protein’s expression would lead to large changes in Treg immunosuppressive activity.
To test the hypothesis, mice were selectively bred to express T cells without the SRC-3 protein using a gene-engineering system called Cre-loxP. With this system, one set of mice is born with three key genetic features: 1) a tissue-specific promoter that indicates where the gene should be read 2) a gene sequence encoding an enzyme called Cre recombinase and 3) a gene sequence encoding a modified estrogen receptor (ER). These animals reproduce with another set of mice that possess the gene of interest flanked by special DNA motifs called LoxP sites. The offspring retains the genetic information from both parents.
To cause the desired gene knockout, the offspring receive an injection of a drug called tamoxifen to liberate the modified estrogen receptor; this, in turn, initiates enzyme activity. Once active, the enzyme recognizes the LoxP sites and snips the DNA at those points to knock out the gene of interest—here, that would be SRC-3 gene in the spleen.
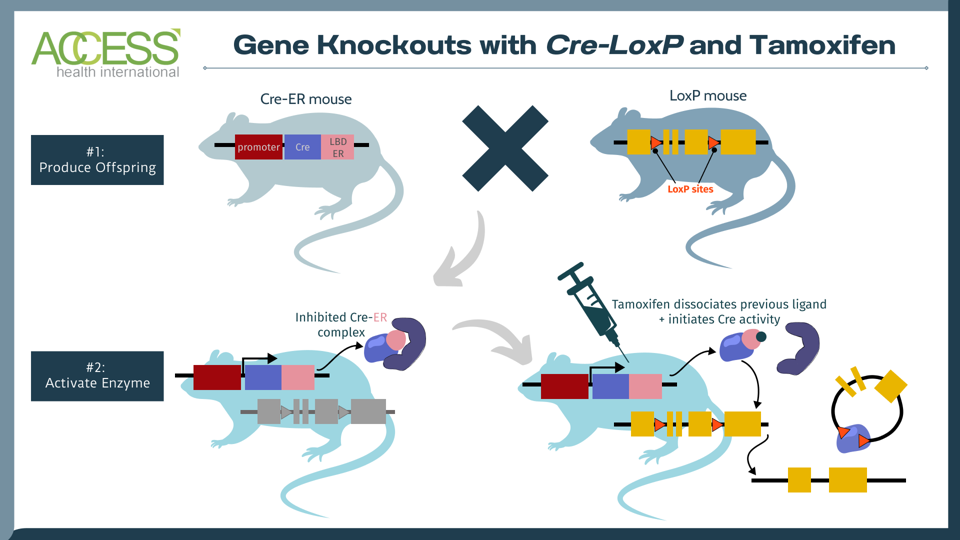
FIGURE 2: The tamoxifen-induced Cre-loxP system is a technique used to knock out a gene of interest in mammals. One set of mice contains the tissue-specific instructions to produce Cre, an enzyme that recognizes a specific DNA sequence called LoxP. Another set of mice is edited to express a gene of interest flanked by LoxP sites. When these two sets of mice reproduce, their offspring contain the genetic instructions for Cre, LoxP and the gene of interest. The Cre recombinase enzyme activates once the offspring receives a tamoxifen injection. The enzyme then recognizes the LoxP DNA sites in the offspring, cuts at these two locations and knocks out the gene of interest. The result: offspring mice without the gene of interest. Abbreviations: LDB ER, estrogen receptor with mutated ligand binding domain.
ACCESS HEALTH INTERNATIONAL
Next, normal mice and knockout mice received an injection of cancer cells to simulate an aggressive breast cancer model. While the normal mice experienced rapid tumor growth, breast tumors were undetectable in the knockout mice 20 days after injection. Further testing revealed that tumors in knockout mice did not return 218+ days later; the authors consider the tumors to be permanently eliminated.
The knockout did not cause the mice to develop systemic autoimmune disease, reproductive dysfunction, shortened lifespans or reduced body weights. This illustrates the promising safety profile for regulatory cells that lack SRC-3.
It is important to note that tamoxifen, the enzyme activator, is necessary to initiate the knockout. When knockout mice with breast cancer either received a vehicle control or tamoxifen, the vehicle control mice developed aggressive breast tumors while the latter demonstrated limited tumor growth.
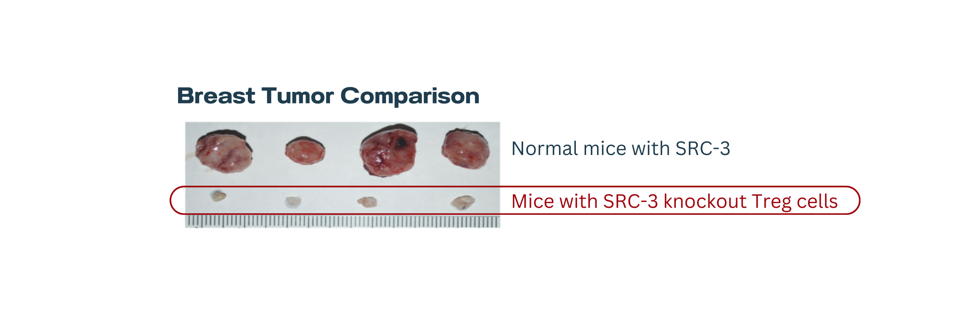
FIGURE 3: Breast tumor comparison.
HAN, S. J., ET AL. (2023).
Tumor Resistance from Adoptive Treg Cell Therapy
An additional experiment evaluated the effect of knockout regulatory T cells on pre-established tumor cells. Here, regular Treg and knockout T reg cells were isolated from the spleens of knockout mice and normal mice respectively. Normal mice with unedited Treg cells and developed breast tumors received either one of the Treg cell injections.
Fascinatingly, mice see a complete and essentially life-long elimination of tumors without adverse effects with a single injection of knockout T cells—despite the fact that normal, immunosuppressive T cells are also present. To eliminate the tumor cells, the adoptive transfer of knockout T cells may also block the immunosuppression of wild-type regulatory T cells. Just as before, the tumor-eradicated mice did not experience tumor recurrence; this suggests that knockout Treg cells encourage a state of long-term tumor resistance. This result is also achieved when normal mice with prostate cancer receive this adoptive Treg therapy.
Dose-response experiments confirm that lower doses of knockout regulatory T cells can suppress tumors, but fail to eradicate them.
Takeaways
Tumors weaponize regulatory T cells to suppress their enemies. However, Treg suppression could be reversed by targeting steroid coreceptor activator 3 in regulatory T cells. Each stage of this study demonstrates a marked difference in tumor size and resistance between knockout mice and normal mice. Without their immunosuppressive qualities, the modified regulatory T cells act like floodgates, allowing a sea of previously hindered immune cells to cast away the solid tumor. Note that the tamoxifen injection used in this study does not affect the tumor; its only effect is to activate the Cre recombinase enzyme and initiate the SRC-3 knockout.
It will be interesting to see if knockout Treg cells behave similarly in humans. A therapy made from modified regulatory T cells could safely treat a wide range of tumors. Additionally, gene-edited Treg therapy may not produce as many autoimmune-like adverse effects as other novel cancer therapies, including CAR T therapy and immune checkpoint inhibitors. The next installment in this series will delve further into the role of SRC-3 in regulatory T cells.
This article was originally published on Forbes and can be read online here.

