Seeking a Solution: Probiotic-Guided CAR T Cells for Solid Tumors
(Posted on Wednesday, November 29, 2023)
3D concept of probiotic cells in intestines.
GETTY
Beyond yogurt ads and health supplements, probiotics could revitalize cell-based cancer immunotherapies such as CAR T therapy. Surprisingly, a paper published in Science demonstrates show how these live microorganisms can guide supercharged T cells into the solid tumor environment—a feat that current iterations of the therapy have yet to overcome. While this method is still under experimentation, it may be a potential answer to this challenge.
CAR T Therapy vs Solid Tumors
At the base of this research lies Chimeric Antigen Receptor T Cell therapy, a revolutionary cell-based treatment for certain blood cancers—this includes large b cell lymphoma, acute lymphoblastic leukemia, and multiple myeloma, among others. For this therapy, white blood cells known as “killer” T cells are drawn from the patient and bioengineered in the lab to carry a synthetic receptor. Once the patient receives the rejuvenated cells, the receptor heightens the killer T cells’ ability to recognize and eliminate a programmed target. It is often regarded as a “living drug,” as the cells multiply in the body and fight cancer.
CAR T therapy can send difficult-to-treat blood cancers into remission, but struggles in the face of solid tumors despite vigorous research efforts. Solid tumors present distinct challenges that traditional CAR T therapy cannot yet address. For example, solid tumors express a wider range of biological tags, or antigens, than blood cells. It is therefore more difficult to identify a specific target for the CAR T cells. What is needed are tumor-associated antigens (TAA), or antigens unique to tumors and not shared by normal healthy tissues. Suppose a CAR T cell is programmed to pursue an antigen found in tumors and healthy tissues alike. In that case, fatal toxicity can occur from the cell correctly attacking the antigen found in healthy tissues. Additionally, solid tumors often exhibit antigen-negative relapse due to selective pressures from targeted therapies.
Probiotics and CAR T Cells (ProCAR)
In the quest to combat solid tumors, people exhibit boundless inventiveness and a relentless commitment to innovation. This is the case for researchers at Colombia University, who created a system that forgoes conventional antigen tags. Rather than rely on antigens to guide the CAR T cells, their CAR T cells depend on probiotics, generally safe bacteria primarily found in the gut, to recognize and siege the tumor. Combining probiotics and CAR T cells (ProCAR) could achieve the tumor-specific targeting that conventional methods lack.
The experimental system relies on three main components: engineered bacteria, a molecular tag, and CAR T cells. First, a known probiotics strain called Escherichia coli Nissle 1917 (EcN) is introduced to the tumor. The bacteria infiltrate the tumor despite the hostile conditions and selectively colonize the tumor site. The bacteria are engineered to equip a synthetic gene circuit; the circuit triggers lysis events, allowing the bacteria to cyclically release genetic information needed to produce the molecular tags—but only once bacteria growth within the solid tumor environment has reached a critical population density.
The tag is composed of a modified green fluorescent protein and another component that binds to molecules found in high abundance on most solid tumors, including collagens and fibronectins. The CAR T cell binds to the green fluorescent protein on the tag, activates its signaling domain, and releases cytotoxic immune chemicals to barrage the solid tumor.
The key to this platform is the tumor-restricted growth of bacteria. In theory, with the tags localized at the tumor core, the CAR T cells should accurately know where to deliver its cytotoxic punch.
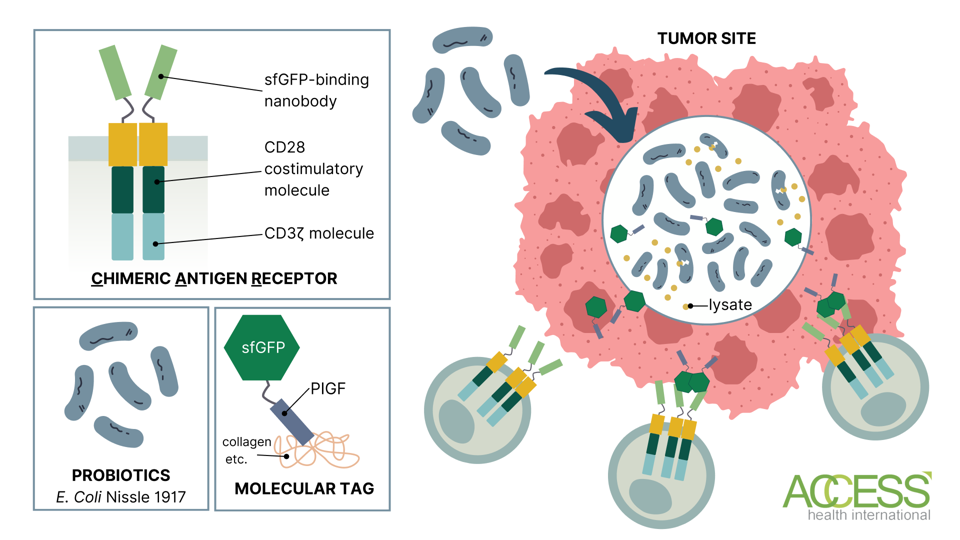
FIGURE 1: Probiotic CAR T therapy. The bacteria produce a molecular tag that the CAR T cells are programmed to target. The chimeric antigen receptor (CAR) is composed of a superfolder green fluorescent protein (sfGFP)-binding nanobody in the extracellular domain and CD28 and CD3ζ molecules in the intracellular signaling domain. The molecular tag bridges the tumor and the CAR T cell. Abbreviations: PlGF-2123–144, placenta growth factor-2
ACCESS HEALTH INTERNATIONAL
ProCAR Demonstrates Proof-of-Concept
How do probiotic-driven CAR T cells perform? The study authors conducted a series of experiments to determine if their ProCAR platform could safely and effectively target solid tumors.
Immunocompromised mice with cancer were given either an intratumoral injection of probiotics that produce the desired tag, probiotics that make green fluorescent protein, or an empty control. The CAR T cells were delivered directly to the tumor 48 hours later. The probiotics that made green fluorescent protein did not slow tumor growth; these proteins lack the binding sites needed to anchor to the tumor. In contrast, the Tag probiotic significantly slowed tumor growth and improved survival without impacting mouse body weight (an indicator of mouse health). Bacteria growth did not extend past the tumor leak into healthy organs days after the initial injection, underscoring the tumor-specific growth of these bacteria.
Mice with intact immune systems were also tested. Here, the ProCar platform successfully slows colorectal tumor progression and induces an adaptive immune response. The result implies that these ProCAR T cells can enhance the immune system’s natural ability to fight cancer. However, this phenomenon appears to be restricted to mouse mice and not as applicable to human cell cultures.
CAR T therapy is usually delivered systemically; when the cells are infused, they circulate through the body to find their target. Could ProCAR cells remain effective if delivered systemically? The authors introduced another mechanism into the bacteria to help the CAR T cells find their mark. These probiotics released the molecular tag alongside a synthetic immune chemical called CXCL16 to directly recruit the CAR T cell to the tumor site. Mice with tumors and a single intravenous injection of CAR T cells displayed reduced tumor growth without decreasing body weight. A comparison illustrated that releasing the immune chemical CXCL16 enhances therapeutic benefit.
Takeaways
Solid tumors have evaded conventional immunotherapies, but probiotic-led CAR T cells may stand a chance. This study showcases that probiotic bacteria can facilitate CAR T cell activity, allowing for accurate and effective targeting of solid tumor cells. Pushing this flexible model further, the researchers also illustrate how this antigen-independent platform can be altered to co-release recruiting immune chemicals. These results suggest a promising method of circumnavigating tumor-associated antigen targeting.
It is not yet known how this experimental combo—probiotics and CAR T therapy—will work in humans. This system will likely require adjustments for clinical translation, as humans are more sensitive to bacterial toxins than mice. Ultimately, this study poses a plausible, new route of investigation, and it’s worth trying every solution to solve the pressing problem of solid tumors.
This article was originally published on Forbes and can be read online here.

