Direct Administration of CRISPR-Cas9: Tools for Cell and Gene Therapy
(Posted on Monday, February 19, 2024)
Newly published research offers a novel technique to address a classic problem.
Several of the latest cell-based gene and cancer therapies suffer similar drawbacks. The first is that cell manipulation occurs outside the body, a lengthy process that requires extracting, purifying, and altering cells in a specialized lab before reintroducing the treatment to the bloodstream. The second risk for donor-derived, allogeneic therapies is tissue rejection; this occurs when the body treats the new cells as a foreign threat. A paper published in Nature Biotechnology provides one of a growing number of solutions to this problem: directly modifying genes inside the person. The goal is to deliver gene-editing tools or synthetic genes straight to the cells, removing the need for external handling.
A New, Targeted Delivery System
In their study, UC Berkeley researchers altered gene delivery methods to improve their targeting abilities. The authors optimize the vehicle design through various cell experiments. They discover that their delivery system displays minimal off-target gene editing into the liver—a common concern for in vivo vectors.
Their platform relies on an industry staple for gene therapies: gutted, nonpathogenic HIV viruses. This study deviates from classic design by fusing preassembled Cas9 proteins into the retroviral structure. Previous tests demonstrate that these CRISPR/Cas9 proteins are fully functional when attached as such.
This upgraded shell needs to find its target. Rather than rely on the virus’s natural ability to infect cells, the team modified the virus by embedding several different monoclonal antibodies on the viral surface. The antibodies direct the viral particle to structures found on specific types of blood cells, thus improving the vehicle’s precision.
Next, the virus must enter the target cell. The team accomplishes this feat using a unique glycoprotein called VSVGmut. The original, unadulterated viral protein causes vesicular stomatitis, a disease rarely found in humans. The mutant form does not react to the same stimuli but retains the virus’s ability to diffuse into human cells. This means that when the target cell takes up the vector through endocytosis, the mutant protein shields the genetic package from degradation until it can empty into the cell’s cytoplasm and be expressed.
The team refers to the final product as an enveloped delivery vehicle or an EDV. The virus-like particle can haul artificial genes, CRISPR-based constructs, or both to programmed targets.
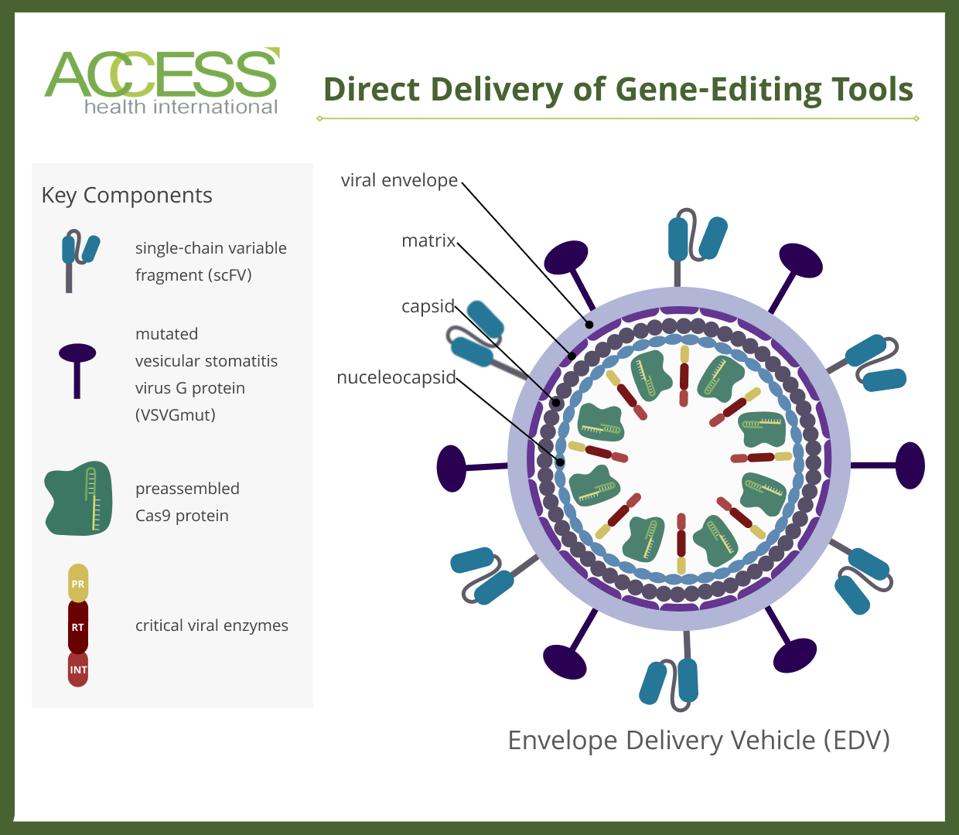
FIGURE 1: Schematic of an enveloped delivery system (EDV). Cas9 proteins are fused to various viral structure proteins. The vehicle carries two unique proteins on its membrane: single-chain variable fragments (scFV) and glycoproteins called VSVGmut. CRISPR/Cas9 gene editors or artificial genes can be placed inside the virus. ABBREVIATIONS: PR, protease; RT, reverse transcriptase; INT, integrase.
ACCESS HEALTH INTERNATIONAL
Creating In Vivo CAR T Cells in Mice
To visualize the practical impact of this technology, the team assessed the vehicle’s performance in living animals. If successful, the vehicle should transform normal white blood cells in the body into supercharged CAR T cells.
This process mimics CAR T therapy, a novel cancer treatment that traditionally extracts a person’s immune T cells to a lab for genetic modification. Conventional methods dictate that the cells must be activated, fitted with synthetic receptors, multiplied and returned to the immunosuppressed patient to wipe out their cancerous blood cells. This costly and time-consuming process could be bypassed entirely if CAR T cells could be made within the body using enveloped delivery vehicles.
To test this, the authors pitted their platform against a lentiviral vector. The enveloped and the lentiviral vector displayed CD3, CD4 and CD28-targeting antibody fragments on their surface to target T cells. Specifically, these elements improve vector cell entry alongside T cell activation and proliferation.
Each vehicle harbored a fluorescent CD19 CAR transgene within. The transgenes in both vectors integrate semi-randomly into the target cell genome. The enveloped delivery vehicle carried an additional Cas9 complex to disrupt T cell receptor alpha constant (TRAC) genes, which ideally should encourage the immune system to accept the modified T cells.
Immunodeficient mice with humanized immune systems received a system infusion of either the enveloped vehicle, the lentiviral vector, or a saline solution as a control. The team analyzed the mouse cells ten days later, looking for signs of chimeric antigen receptors and permanent genome change.
Evidence of Gene-Editing
The lentiviral and enveloped delivery vectors successfully transformed a percentage of T cells into CAR-bearing T cells. More CAR T cells were produced from the lentiviral vectors than the enveloped delivery vectors. The mice from both treatment cohorts did not lose weight and were not detected in liver cells, suggesting the infusions were well tolerated. However, only the enveloped-treated mice demonstrated modified alleles in their CAR+ T cells, a positive sign of in vivo genome editing. As expected, CAR T cells were not found in the control mice.
The CAR-transduced T cells can kill CD19+ B cells as designed. CD19+ B cells are wiped out in lentivirus-treated mice, a result which is likely attributed to a higher number of CAR T cells generated during initial in vivo transduction. Mice treated with the proprietary delivery vehicle displayed varying levels of CD19+ B cells, suggesting that the CAR T cells transduced by the enveloped platform can kill to an extent.
Future Implications
The technology showcased in this paper takes a significant step forward for cell and gene therapies. The particle-like vehicle exhibits notable adaptability, gene-editing precision and minimal liver toxicity. Future research avenues could explore other cell targets to improve vector targeting or investigate novel methods that transition semi-random integration to more precise integration of transgenes into the host genome.
Importantly, this work joins a body of developing in vivo delivery advances aiming to transform the gene therapy landscape, including non-enveloped viral vectors, liposomes, and lipid nanoparticle technology popularized during the COVID-19 pandemic. Engineering cells internally rather than externally in a lab should streamline labor-intensive production and give way to an era of more affordable cell-based treatments. It could also decrease risks associated with tissue rejection or immunosuppressive chemotherapy, a standard yet risky procedure many must undergo to prepare for ex vivo cell infusions.
This story was originally published in Forbes, and can be read online here.

