Molecular “Super Glue”? How Our Body Repairs Broken DNA
(Posted on Friday, March 1, 2024)
We don’t exactly know why we age; we know what aging looks like —the “symptoms”, so to speak— but the root causes remain foggy. One leading hypothesis is that the changes associated with old age, both external and internal, are a result of accumulating DNA damage. As this damage builds, cellular functions begin to break down and important pathways start going haywire.
One of the most extreme forms of DNA damage is the double-strand break, which happens when a strand of DNA snaps in half, leaving two separate slivers floating around. Left unfixed, these strands can snag at and break chromosomes, leading to diseases like cancer and other disorders. But how the body repairs this kind of wreckage has been a source of mystery. Now, scientists at the Dresden University of Technology have managed to shine a light on the process. Published in Cell, their work offers important new insights that may eventually help treat, and possibly reverse, DNA damage.
PARP1: A Protein “Superglue”?
Each cell in the body experiences roughly 100,000 DNA lesions every single day. These all need fixing. A key player in the repair process is an enzyme called PARP1 (Poly [ADP-ribose] polymerase 1), which patrols strands of DNA looking for sites of injury. As soon as it locates a section of damaged DNA, it sends out an emergency flair, rallying other repair proteins into action. Think of PARP1 as a first responder whose job it is to uncover signs of damage, report them, and then rope off the area until backup arrives.
What remained unclear, until now, was how PARP1 helps fix double-strand breaks — in particular, what keeps the two DNA strands from “floating” apart post-break?
One of the reasons for the uncertainty is that studying double-strand breaks can prove difficult; with DNA tucked away in the nuclei of cells, it is tricky to get a good view. And there are so many moving parts that it is hard to tell which enzymes are involved in the repair efforts and which are involved in other routine tasks.
To overcome this issue, the researchers used a number of cutting-edge laboratory techniques to recreate and isolate double-strand DNA breaks in a test tube, entirely independent of cells. This helped grant them a clear field of vision over the entire process. To their knowledge, this is the first time a double-strand break scenario has been recreated outside of cells.
The first step in the timeline, as mentioned, is the initial discovery of DNA damage by PARP1. Next, PARP1 calls other PARP1 molecules to the site of injury and, once enough of the molecules have gathered, they connect to one another and connect to the loose ends of the broken DNA strands. The result is very similar to that of superglue: a sticky mass that keeps things fixed together. The mix of PARP1 and DNA, referred to as condensate, also acts as a special healing zone by fencing the area off from the rest of the cell.
Once PARP1 has secured the broken strands of DNA, it switches gears and jumps into enzyme mode, calling out to various other repair proteins to make their way over. One of these proteins is RNA-binding protein FUS/TLS, or just FUS for short. Prior to these new findings, the precise function of the protein was unknown; it was clearly important to repair, since it crowded around sites of damage, but researchers hadn’t yet managed to establish what it does. With the improved “visibility” of the test tube environment, the scientists found out that it acts as an adhesive remover of sorts. Since the other repair proteins need access to the DNA, the PARP1 molecules need to be moved out of the way. FUS helps to “soften the glue”.
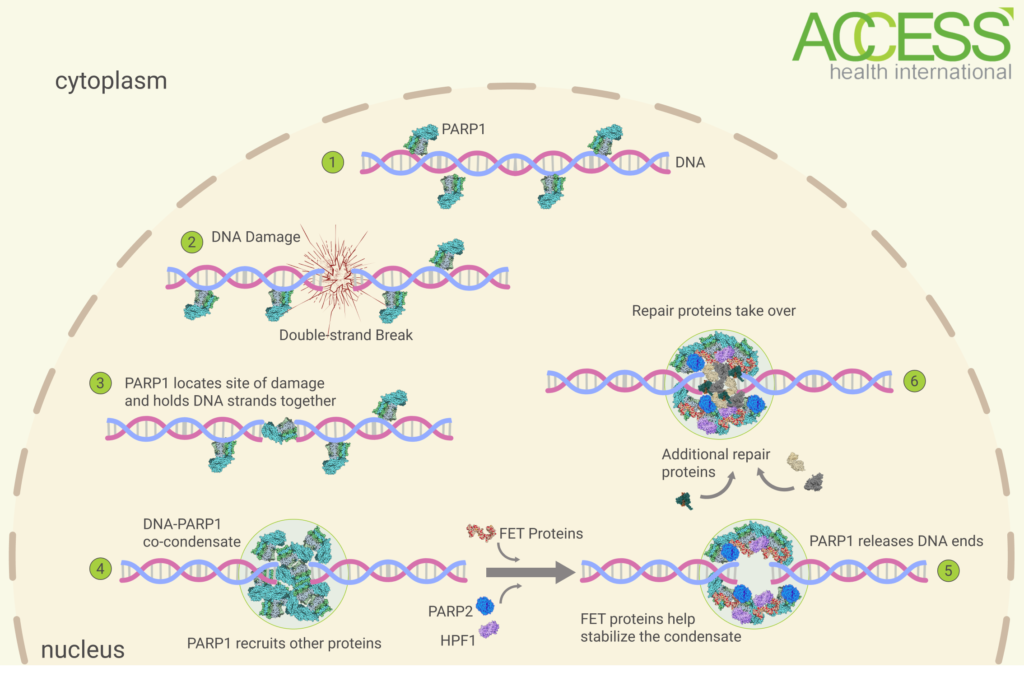
FIGURE 1. Abbreviations — PARP1 = Poly [ADP-ribose] polymerase 1; FET Proteins = FUS, EWSR1 and TAF15 Proteins; PARP2 = Poly [ADP-ribose] polymerase 2; HPF1 = Histone PARylation Factor 1. Source: ACCESS Health International
Implications & Takeaways
DNA damage contributes to aging. PARP1 is a crucial component of the DNA repair machinery. Indeed, a study of centenarians revealed a strong positive correlation between lifespan and PARP1 activity in lymphocytes. The same was found in an analysis of 13 other mammalian species, with heightened PARP1 activity correlating with longevity.
Although the current research doesn’t carry any immediate therapeutic weight, it does offer novel insights into the nuances of PARP1 activity and the DNA repair process more generally. Insofar as these are both closely linked to aging and life span, the more we understand the better. Knowing how PARP1 works may open up doors for the development of drugs that mimic the enzyme and stimulate DNA repair.
The study is also important for being the first of its kind; no other team has yet to recreate double-strand DNA breaks outside of the cellular environment. The researchers’ detailed description of their methodology will undoubtedly facilitate other breakthroughs in the future.

