A Better Concept For mRNA Vaccines: Self-Amplification
(Posted on Monday, April 29, 2024)
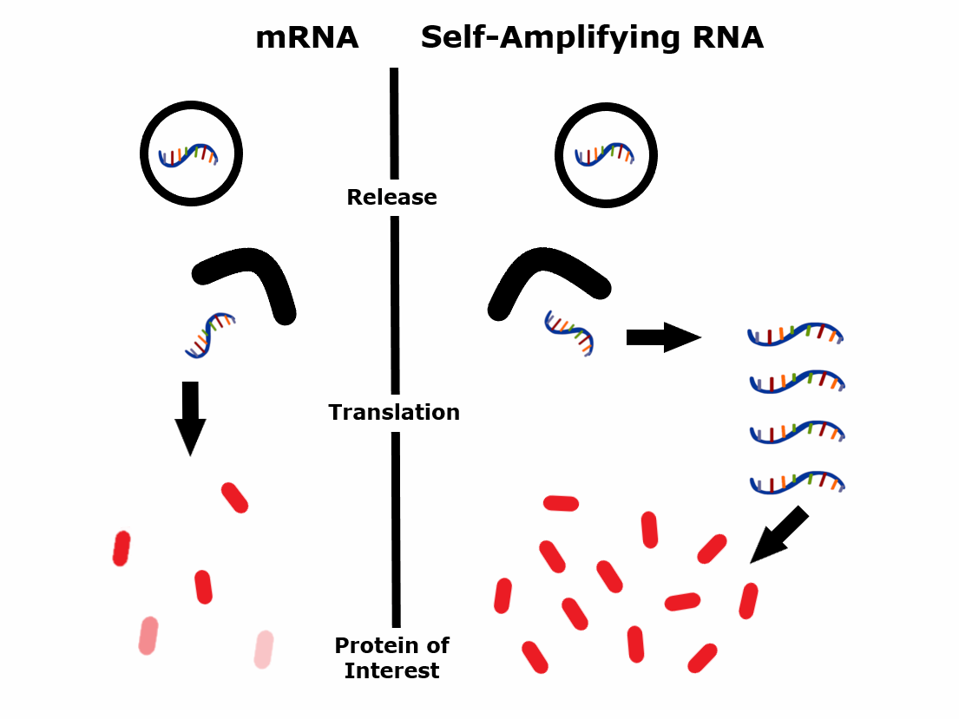
Schematic representation of how self-amplifying RNA works.
This article was originally published on Forbes on 4/29/2024.
Despite the apparent success of Covid mRNA vaccines, fundamental issues remain. The limitations of the current Pfizer and Moderna vaccines include short lifetime, substantial cold chain requirements, and lack of mature B and T cell responses due to short-lived synthesized proteins. Two newly reported vaccines using a self-amplifying RNA technology may overcome these problems: GEMCOVAC-OM and ARCT-154. These two mRNA vaccines herald a new era of vaccination for coronaviruses. This article delves into the unique mechanisms of self-amplifying messenger RNA vaccines based on promising results from recent human trials.
Traditional mRNA vaccines contain a linear RNA instruction with pseudouridine modified nucleotides. When the mRNA enters your cells, they produce a protein that, if properly administered, induces an immune response.
Self-amplifying RNA vaccines contain an additional genetic material that encodes an enzyme called a “replicase.” This replicase can create multiple copies of the original self-amplifying RNA strand based on a viral RNA carrier that expresses numerous copies of an RNA specifying a chosen protein.
When the self-amplifying RNA vaccine enters your cells, it triggers the activation of the replicase gene. The replicase produces many copies of the RNA, resulting in significantly higher target protein levels than a regular mRNA vaccine.
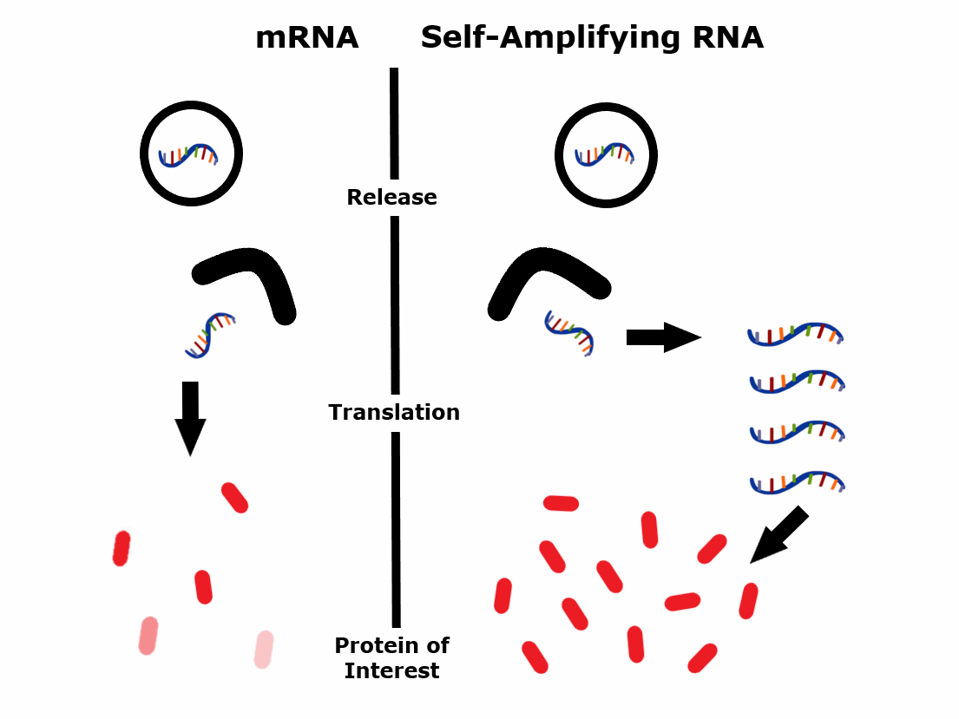
FIGURE 1: Schematic representation of how self-amplifying RNA works.
The first-generation mRNA vaccines produce a short-lived mRNA with few antigen proteins lasting only 2-3 days. Self-amplifying RNA produces many more proteins from a much smaller RNA input, which lasts 20-26 days. The self-amplifying nature of self-amplifying RNA also helps stimulate the innate immune system, acting as a built-in adjuvant to boost the immune response further.
Finally, due to their self-amplification property, self-amplifying RNA vaccines can achieve the same and sometimes better levels of immune response with lower doses than traditional mRNA vaccines.
GEMCOVAC-OM and ARCT-154 are two self-amplifying RNA vaccines that are similar in that they achieve the same goal of being highly effective, long-lasting vaccine candidates, but they differ slightly in some characteristics.
GEMCOVAC-OM was developed by Gennova Biopharmaceuticals in Pune, India. In Nature, Dr. Amit Saraf and colleagues demonstrated the vaccine’s capabilities in a randomized control human trial involving over 3,000 participants. They tested the efficacy of GEMCOVAC-OM versus the AstraZeneca ChAdOx1 nCoV-19 vaccine. They found that their vaccine had significantly higher anti-Omicron IgG antibody responses (93.0%) than the AstraZeneca vaccine (76.7%) 29 days after vaccination.
We note, however, that these studies were performed against Omicron BA.1, a strain of the virus that has long since been overrun by more infectious and immune-resistant strains. We encourage those behind GEMCOVAC-OM to continue human testing against more current strains of SARS-CoV-2.
ARCT-154 is a similarly impressive self-amplifying RNA vaccine recently approved in Japan. Described by Dr. Yoshiaki Oda and colleagues in The Lancet, the vaccine developed by Arcturus Therapeutics conveyed similarly impressive results over the Pfizer BNT162b2 vaccine in a study of over 800.
Over six months, anti-Covid antibody titers conveyed by ARCT-154 (in red below) significantly outperformed those of the Pfizer vaccine (in blue below). Additionally, the results seen above are against Omicron BA.4/BA.5, which are more similar to current strains of SARS-CoV-2 circulating today.
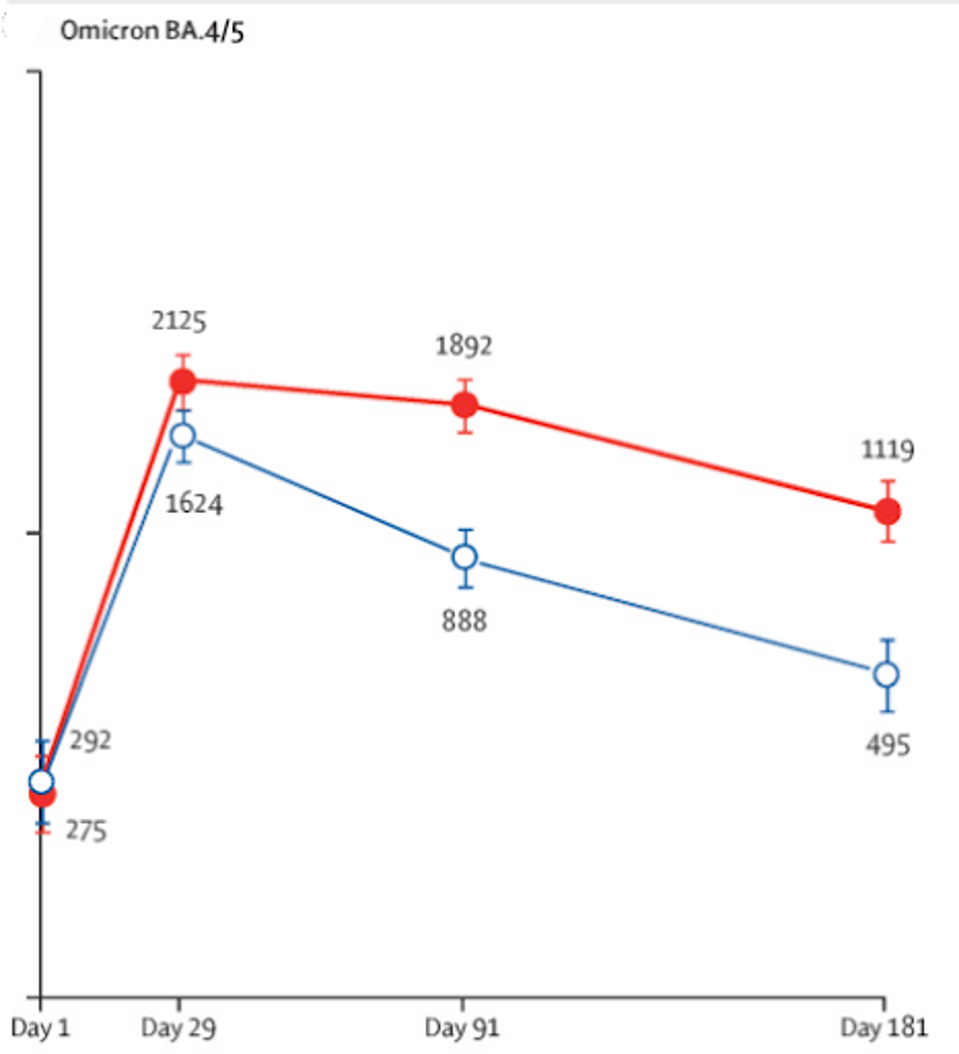
FIGURE 2: Geometric mean titers of surrogate neutralizing antibodies against the SARS-CoV-2 Omicron BA.4/5 up to 6 months after vaccination with one booster dose of either ARCT-154 or BNT162b2.
GEMCOVAC-OM and ARCT-154 also have the distinct advantage of being lyophilized, which is the freeze-drying of the vaccine delivery mechanism. This enables vaccine stability at 2-9 degrees Celsius for up to 12 months without using super-cold storage systems. This stability allows for their stockpiling for future use. Some lyophilized preparations may be stable at room temperature for many months.
GEMCOVAC-OM uses an anionic particle, whereas ARCT-154 uses a proprietary lipid nanoparticle called LUNAR®. Both systems enable low-resource countries to access vaccines by eliminating cold chain storage requirements, greatly expanding their utility.
Another advantage is that self-amplifying RNA vaccines do not require modified nucleotides like pseudouridine. Recent studies have shown that ribosomes may misread the correct protein sequence at sites of pseudouridine insertion, producing aberrant peptides that may induce auto-immune responses.
Another difference between the two vaccines is administration. ARCT-154 is injected intramuscularly, whereas GEMCOVAC-OM is applied intradermally without a needle through the skin via Powerjet Injector.
Intradermal delivery presents the antigens more naturally than intramuscularly, as the skin is loaded with antigen-presenting cells, whereas the muscles are not. Presentation in the skin in conjunction with a long duration of expression may yield a much more mature B cell response, as well as CD-4, CD-8, and T memory cells.
These two reports are encouraging for mRNA vaccine development for many viruses as they offer the following advantages:
(1) the use of small amounts of RNA;
(2) the lack of requirement for altered nucleotides
(3) the longer production of antigen maturation;
(4) the potential maturation of B cells, T cells, and memory cells;
(5) the long-term stability; and
(6) the hope that these vaccines will offer a blueprint for broader, more durable protection against various diseases.
Read the original article on Forbes.

