A Closer Look At Protein-Based Therapies For Dry Macular Degeneration
(Posted on Thursday, August 10, 2023)
Originally published on Forbes on 8/4/2023

This story is part of a series on the current progression in Regenerative Medicine. This piece is part of a series dedicated to the eye, and it marks the second of a three-part series on dry age-related macular degeneration. Future pieces in the series include stem cell and gene therapies and omega-3 for macular degeneration.
In 1999, I defined regenerative medicine as the collection of interventions that restore to normal function tissues and organs that have been damaged by disease, injured by trauma, or worn by time. I include a full spectrum of chemical, gene, and protein-based medicines, cell-based therapies, and biomechanical interventions that achieve that goal.
Dry age-related macular degeneration is a significant medical issue. Dry age-related macular degeneration and geographic atrophy have an economic impact of over $22 billion in the USA alone. Age-related macular degeneration affects a staggering number of people worldwide. Over 200 million individuals globally and nearly 20 million adults in the US alone are impacted by this condition, making it more prevalent than glaucoma and dry eye combined.
Macular degeneration is under intense study, with numerous resources dedicated to understanding its substantial impacts. In Clinical Ophthalmology, a review examined the investigational therapeutics for treating dry age-related macular degeneration, including protein-based therapies.
No approved protein-based therapies for treating dry macular degeneration exist, but ongoing research and clinical trials hold promise. Scientists are studying potential protein-based treatments such as antioxidative therapy and complement cascade inhibition therapy.
What is Dry Age-Related Macular Degeneration?
Dry age-related macular degeneration is a progressive condition characterized by the gradual deterioration of the macula, the area responsible for sharp central vision. As the cells in the retina in this area die and are not regenerated, visual acuity deteriorates progressively.
The dry subtype is the most common form for about 80% of all macular degeneration cases. It occurs when tiny yellow deposits known as drusen accumulate beneath the retina. These drusen are waste products that gradually enlarge, leading to the death of retinal cells in the macula and resulting in blurred vision. The progression of the disease typically unfolds slowly over time, with three stages: early, intermediate, and late.
Unfortunately, there is currently no treatment available for late-stage dry macular degeneration. However, maximizing the remaining vision and protecting the unaffected eye is possible if only one eye is affected. Symptoms may include difficulty reading, engaging in crafts, cooking, recognizing faces, and driving. Vision may be affected in low light, and straight lines might seem distorted or absent.
Today, researchers and practitioners are exploring diverse therapeutic approaches to manage and study dry macular degeneration effectively.
Current Therapies for Dry Macular Degeneration
Antioxidative therapy is a widely accepted treatment for age-related macular degeneration. This therapy aims to decrease oxidative stress and inflammation in the retina, contributing to dry macular degeneration. Antioxidative therapy focuses on improving retinal defenses against oxidative damage through the dietary intake of antioxidants, developing antioxidant-based drugs, and gene therapies.
Antioxidants neutralize harmful free radicals produced by the body and reduce oxidative damage in cells. They can be enzymatic or non-enzymatic. Enzymatic antioxidants include proteins like superoxide dismutase, catalase, and glutathione peroxidase. Each plays a role in the body’s natural defense against oxidative stress.
Antioxidative therapy in relation to age-related macular degeneration focuses on improving retinal defenses against oxidative damage through the dietary intake of antioxidants, the development of anti-oxidant-based drugs, and gene therapies. This type of therapy is a broader range of approaches and includes more than just protein-based therapies; the complement cascade inhibition therapies, on the other hand, are more specific.
A Complex Complement Cascade
The complement system and its related cascade are vital to the immune system. It consists of proteins that activate a response when the body encounters an antigen. The complement system has three major pathways: the classical, alternative, and lectin pathways. About half of all dry age-related macular degeneration patients have gene mutations that affect complement regulation, particularly in the genes involved in the alternative pathway.
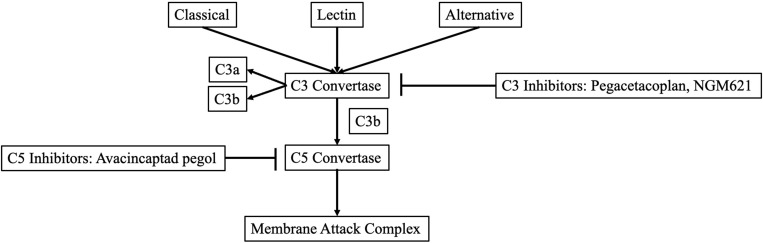
All the pathways converge and form a C3 convertase, breaking into C3 and C3b. C3 is involved in tissue damage and inflammation, while C3b is needed to create C5 convertase. C5 is an inflammatory molecule. When C3, C3b, and C5 team up, they make a membrane attack complex or MAC. This MAC damages cells and attracts cells to form swelling, which is necessary for the immune response.
The imbalance of the complement system is a significant factor in age-related macular degeneration. However, developing complement inhibitors for macular degeneration treatment is challenging due to the complexity of the complement system and the variability in individual responses to these inhibitors.
Ongoing research and clinical trials actively explore the potential of complement inhibitors as a viable therapeutic approach for dry age-related macular degeneration. The current focus revolves around rigorously evaluating the effectiveness of complement inhibitors. This evaluation requires robust and sensitive measures to gauge disease progression and treatment response.
The design of clinical trials carefully considers these factors in conjunction with outcome measures. Despite the challenges, continuous research and clinical trials persist in investigating complement inhibitors as a promising therapeutic option for dry age-related macular degeneration.
Research on Complement Inhibitors for Macular Degeneration
Nearly 40 clinical trials have examined or are investigating a minimum of 14 distinct complement inhibitors for age-related macular degeneration. Unfortunately, several of these trials have yielded disappointing results. For instance, lampalizumab, an anti-factor D antibody, did not meet expectations in two phase III trials focused on geographic atrophy. However, amidst these setbacks, two complement inhibitors, namely APL-2 and Zimura, have exhibited remarkable efficacy in inhibiting the progression of geographic atrophy during phase II clinical trials.
APL-2 is also known as pegcetacoplan and works by inhibiting the C3 complement. In a phase II trial, APL-2 showed promising results by reducing the growth rate of geographic atrophy by 29% monthly and 20% every other month compared to the placebo group.
Zimura, on the other hand, acts as a C5 inhibitor. When patients received a monthly dosage of Zimura along with anti-VEGF, approximately 60% experienced a visual acuity improvement of three lines or more. This outcome is better compared to those who received anti-VEGF alone.
Zimura is unique because it is effective for both wet and dry macular degeneration subtypes. The effectiveness of Zimura as a new medication for addressing dry age-related macular degeneration will rely on the outcome of the ongoing phase III clinical trials.
What Comes Next
Millions of people worldwide are affected by dry age-related macular degeneration. However, researchers are working tirelessly on developing protein-based therapies and complement inhibitors to help combat this condition. While it is challenging due to the complexity of the complement system and varied responses to the inhibitors, ongoing research and clinical trials are showing promising results. With the positive outlook for treatment and continued progress, there is hope that those suffering from dry age-related macular degeneration will soon have a dependable treatment option.
To learn more about the eye, read more stories at www.williamhaseltine.com