A Deeper Look at Anti-PD-1/PD-L1 Checkpoint Inhibitors for Cancer
(Posted on Wednesday, June 5, 2024)
In a previous story, I described pembrolizumab, a PD-1 targeting checkpoint inhibitor, in some detail. Since its launch, several companies have introduced antibodies targeting the same receptor. This article summarizes some of these recent advances and how they work.
Fight Cancer with Checkpoint Inhibitors
When many cancers advance, spread and reoccur, checkpoint inhibitors can help keep difficult tumors at bay. These antibody-based immunotherapies can restore the body’s ability to recognize and eliminate cancer cells—an approach that often pairs well with other treatment options, including surgery, chemotherapy and radiation.
The antibodies are delivered intravenously. Once in the blood, these antibodies block molecules called checkpoint proteins on immune T cells. This blocking interaction restores the immune system’s ability to recognize and kill cancer cells.
While not all inhibitors are crafted alike, around half of the inhibitors currently on the market share the same immune target: a protein known as PD-1 found on white blood T cells. This strategy demonstrates clinical efficiency for many cancers, including melanoma, lung, and head and neck cancers.
What Immune Checkpoints Do
Immune checkpoints lie at the center of this mechanism. This naturally occurring system helps the immune system maintain self-tolerance—the body’s ability to recognize self against aberrant proteins that arise from cancer or infections —and minimize immune response damage to healthy tissues.
One key immune checkpoint is known as PD-1, or Programmed Cell Death Protein-1. This receptor is found on the surface of T cells and other immune cells. These proteins protect the body from overly strong immune responses and autoimmunity. When PD-1 binds to its partner receptors on other cells, PD-L1 or PD-L2, it sends a signal to the T cell to paralyze its normal immune function.
Block PD-1 Checkpoints, Shrink Tumors
The immune system is equipped to suppress tumors on a small scale. Cancer cells overcome this anti-tumor activity by producing molecules to inhibit immune cell killing. By over-expressing PD-L1 checkpoint proteins, cancer cells can turn off T cells that would otherwise attack the tumor.
Enter anti-PD-1 checkpoint inhibitors. These therapies bind antibodies to PD-1 proteins, preventing the PD-1 protein from binding to its ligands, PD-L1 or PD-L2. The resulting T cell is freed of its restraints and can sustain its activity in the tumor microenvironment. In a previous story, I described pembrolizumab, a PD-1 targeting checkpoint inhibitor, in some detail. Since its launch, several companies have introduced antibodies targeting the same receptor. This article summarizes what they are and how they work.
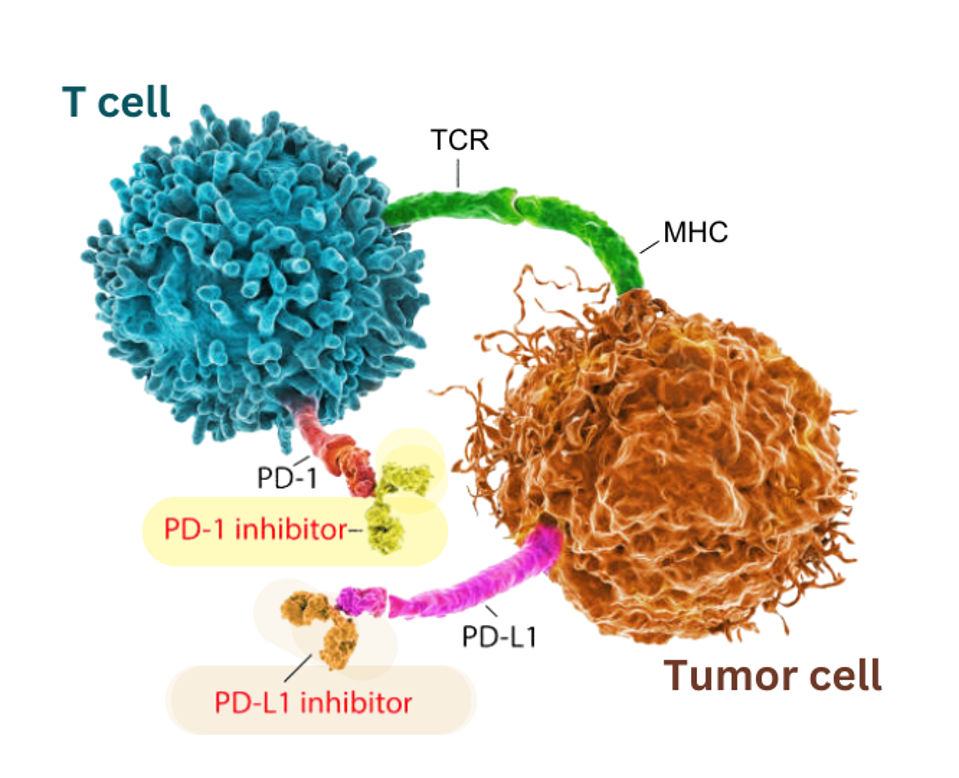
Checkpoint inhibitors boost anticancer activity by targeting immune checkpoint proteins. The antibodies here block two different checkpoint proteins: PD-1 on T cells (highlighted in yellow) and its corresponding ligand, PD-L1 on tumor cells (highlighted in orange).
ADAPTED FROM GETTY
Anti-PD-1 Checkpoint Inhibitors
Checkpoint inhibitors use antibodies to bind to and prevent checkpoint protein interactions. Currently, there are seven federally approved checkpoint inhibitors that block PD-1 checkpoint receptors. Each inhibitor benefits a distinct array of cancers. For example, pembrolizumab, sold by Merck & Co., is indicated to treat over 18 different types of cancers. In comparison, BeiGene’s tislelizumab only gained FDA approval to treat patients with a certain type of esophageal cancer.
These drugs can be administered alone as a monotherapy or with other cancer treatments, including inhibitors targeting different checkpoint proteins and chemotherapy. The characteristics of the cancer at hand will dictate how each inhibitor is used.
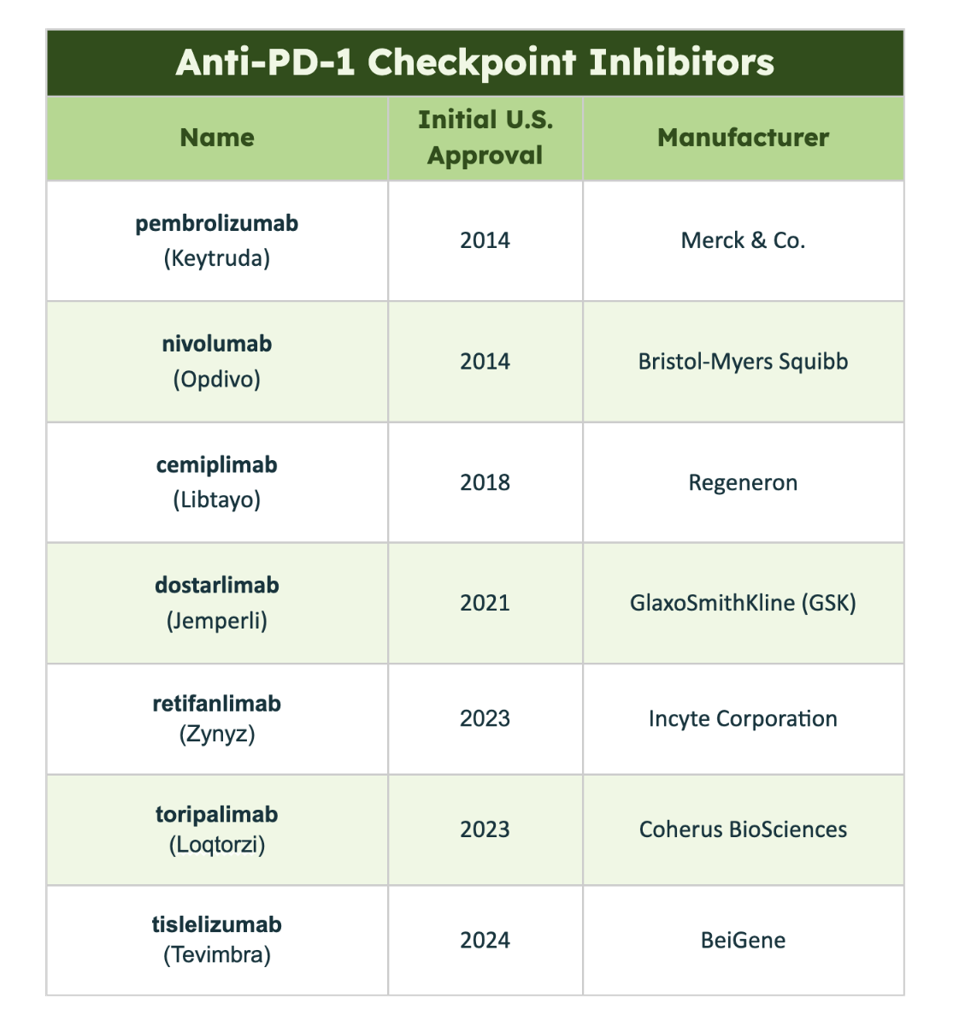
Table 1: List of all anti-PD-1 checkpoint inhibitors approved in the United States as of May 2024.
ACCESS HEALTH INTERNATIONAL
Adverse Effects
Although successful in treating several cancers, anti-PD-1 therapies can trigger several adverse effects. Most reactions occur as a consequence of unleashing immune activity, resulting in a variety of issues. Most notably, the inhibitors can stoke inflammation throughout the body, such as on the skin or in the lungs, colon or liver. These inhibitors can also tamper with hormone levels in the thyroid and adrenal glands.
The skin can turn itchy or red with a rash. Issues with lung inflammation, or pneumonitis, often cause coughing, shortness of breath, and fever. Patients with colitis typically experience frequent diarrhea, abdominal pain, nausea, vomiting, fatigue or decreased appetite. Liver and kidney dysfunction can similarly cause nausea, fatigue or loss of appetite. Patients also commonly report feeling pain in the joints, back, and/or muscles.
Though these adverse events can range from mild to life-threatening, checkpoint inhibitors are generally considered more tolerable than chemotherapy and radiation. Clinicians monitor each patient to prevent these reactions from becoming life-threatening and are often successful.
Targeting Partner Receptors PD-L1 & PD-L2
Originally, checkpoint inhibitors could only target T cells to interfere with the PD-1 immune checkpoint axis. Now, it is possible to interrupt PD-1 interactions by targeting PD-1’s partner receptors on other cells.
PD-1 possesses two known binding partners: PD-L1 and PD-L2. These proteins are often over-expressed on tumor cells. Though there are federally approved checkpoint inhibitors for the former, the clinical potential for the latter is still under investigation.
Three anti-PD-L1 inhibitors are available for use: atezolizumab (Tecentriq), durvalumab (Imfinzi), and avelumab (Bavencio). They emerged a few years after the FDA approved the first anti-PD-1 inhibitor.
Interestingly, though these inhibitors target the other side of the PD-1 axis, they do not treat exactly the same diseases as their anti-PD-1 counterparts. Certain cancers, such as a kind of soft tissue tumor called alveolar soft part sarcoma and small cell lung cancer, have only shown improvement under anti-PD-L1 inhibitors. For other cancers, including non-small cell lung cancer, hepatocellular carcinoma, and melanoma, either type of inhibitor could elicit clinical benefit. The decision between which inhibitor to choose will depend on several factors, such as the available efficacy data for the disease and the patient’s previous treatment history.
Potential Future Research
The full potential for anti-PD-1 checkpoint inhibitors remains untapped. Investigational inhibitors will continue gaining approval and expanding this inhibitor mechanism’s applicability. However, an important caveat exists. Although these drugs can help suppress difficult-to-treat tumors, their effect is not consistent across different cancer types; some tumors respond better than others. As a result, improving drug safety profiles and overall clinical benefit are major research focuses for the field.
Clinical trials administering anti-PD-1 inhibitors alongside other known cancer treatments, such as chemotherapy, are pivotal in understanding how best to deliver this therapy. While combining distinctly-targeting inhibitors is possible, an anti-PD-1 and anti-PD-L1 inhibitor regime has yet to be approved. With more investigation, anti-PD-L1 and anti-PD-L2-targeting therapies could one day join this pool, as well.
Takeaways
Checkpoint inhibitors represent one of the most significant advances in cancer treatment over the past twenty years. While they are not the answer to all cancers, their impact is profound, especially when used in combination with other treatments such as therapy, chemotherapy, and emerging cell-based therapies such as CAR T Therapy. This is just the beginning; as research continues and new checkpoints are discovered, the potential of these therapies will only grow, offering greater hope for cancer patients.
This article joins a growing series on mono cancer treatments, including novel immunotherapies such as CAR T therapy and checkpoint inhibitors. Find more at www.williamhaseltine.com.
Read the article online on Forbes.