The Way That Covid-19 Tricks The Immune System Could Result In More Severe Illness
(Posted on Wednesday, May 27, 2020)

Chief Scientific Officer Dr Jeff Drew, uses a microscope to look at cells containing the novel coronavirus SARS-CoV-2 in the Stabilitech laboratory in Burgess Hill south east England, on May 15, 2020 where scientists are trying to develop an oral vaccine for the COVID-19 illness. – The scientists at Stabilitech are one of the teams attempting to develop a vaccine for COVID-19. Ingested in a capsule into the gut, Stabilitech’s potential oral vaccine aims to prompt an immune response in mucosal cells in the respiratory system and elsewhere in the body. The firm believes that will be more effective in tackling respiratory illnesses like coronavirus. The British government is touting the country as a global leader in the big-money investment race to find a vaccine for COVID-19. (Photo by BEN STANSALL / AFP) / TO GO WITH AFP STORY BY JOE JACKSON (Photo by BEN STANSALL/AFP via Getty Images)
AFP VIA GETTY IMAGES
The virus that causes Covid-19, SARS-CoV-2 (SARS-2), has a nasty trick up its sleeve.
Upon infection, most viruses trigger a vigorous immune response from both arms of the immune system: a lymphoid response and a myeloid response.
Interferons released from infected cells trigger the lymphoid response, resulting in antivirus antibodies which bind to and eliminate the virus from the body. T cells are also activated that can recognize and eliminate infected cells.
The myeloid pathway works differently. Myeloid cells attack the virus and the infected cells directly. Some myeloid cells engulf and destroy virus particles, others kill the infected cells directly, and others still induce a protective inflammatory response by release of compounds called cytokines.
The way SARS-2 affects both arms of the immune response is different from other viruses. The lymphoid pathway is muted and the myeloid pathway hyperactive. This helps explain why some people who recover from Covid-19 have very low, sometimes undetectable levels of anti-SARS-2 antibodies, and others have undetectable levels of “neutralizing” antibodies capable of the inactivating virus in laboratory experiments.
Failure to induce high levels of protective antibodies may also be related to a poor memory of prior infections. The upshot is the real possibility that protection following infection may be transient. We know that people who recover from coronavirus colds can be reinfected by the same strain of virus a year later. We will learn over time whether that is true of Covid-19 as well.
A hyperactive myeloid response, on the other hand, can result in the famous cytokine storm associated with the rapid decline and death of Covid-19 patients. The two phenomena may in fact be linked. An ineffective or weak lymphoid response may lead to prolific virus replication. Large amounts of virus can in turn trigger a violent myeloid response, in some cases violent enough to kill.
Detailed studies reveal how SARS-2 virus pulls off this trick. In addition to the genes the virus needs to reproduce—the replicative enzymes and the proteins that comprise the outer and inner shells the virus—coronaviruses produce many other proteins. They all go by the generic name open reading frame gene (orf), followed by a number and sometimes an additional letter to distinguish one from the other. That’s because we don’t know much about them or what they do, besides the fact that most aren’t needed to grow the virus in a test tube.
This reminds me of my earlier work with HIV-1, the virus that causes AIDS. My Harvard group discovered six such proteins. Two were absolutely required for virus replication in a test tube, but the other four were not. Years of extensive study revealed that in fact, these proteins provided HIV-1 with a bag of tricks that allowed it to survive not only in the test tube, but in the much more rigorous environment of our bodies. I’ll bet dollars to donuts that a similar focus on the so called “accessory genes” of the Covid-19 viruses will reveal another set of tools they use to do their dirty work. And there are a lot of them. After all, SAR-2 contains three times the amount of genetic information as HIV-1.
We have a hint of how one of those genes, named orf3b, works. Initial studies of SARS-1, the virus that caused the earlier SARS epidemic, reveal that the protein made by the orf3b down regulates the production interferons needed to trigger the first arm of the immune system—the lymphoid pathway responsible for producing antibodies and killer T cells.
A recent study shows that the orf3b protein of SARS-2 does the same thing, only better. The protein made by orf3b in most strains of SARS-2 is shorter and more potent in tamping down the interferon response than that of SARS-1. That may be one of the reasons SARS-2 can be transmitted more efficiently than SARS-1. A weaker antibody response means than more virus particles are made.
There is an ominous twist to the story. Two strains of SARS-2 isolated from very ill patients carry yet a third variant of orf3B. This variant is longer than that of the original SARS-1 gene. When tested, it turns out to be more potent than either in its ability to compromise the interferon response. The implication? This may be one of the first signs that a more transmissible and lethal strain of SARS-2 will emerge, one that accelerates the pandemic still further. Watch this space!
In dampening the antibody response to infection and ramping up production of chemokines, SARS-2 is amplifying what happens to us naturally as our immune systems age. While our ability to mount an effective antibody and T cell response to new infections declines, the myeloid arm of the immune system becomes overactive. These features of the aging immune system account for both the decline in our response to new vaccines and to an increase in inflammatory auto-immune disease such as rheumatoid arthritis.

Blood cell generation from the pluripotent stem cell showing predisposition toward the myeloid lineage with aging.
LORD JM. THE EFFECT OF AGEING OF THE IMMUNE SYSTEM ON VACCINATION RESPONSES. HUM VACCIN IMMUNOTHER. 2013;9(6):1364‐1367. DOI:10.4161/HV.24696
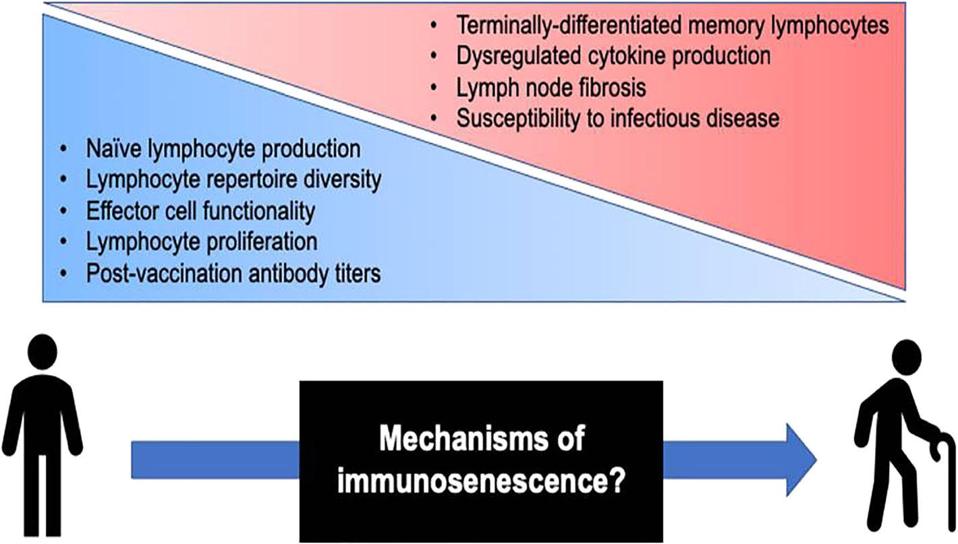
Immunological changes associated with aging and adaptive immunosenescence.
CROOKE SN, OVSYANNIKOVA IG, POLAND GA, KENNEDY RB. IMMUNOSENESCENCE AND HUMAN VACCINE IMMUNE RESPONSES. IMMUN AGEING. 2019;16:25. PUBLISHED 2019 SEP 13. DOI:10.1186/S12979-019-0164-9
In other words, infection by SARS-2 tips the balance of a mis-regulated immune system still further, explaining what we know all too well: older people are at far higher risk of serious disease and dying from Covid-19 than the young.
Every day we learn more about this virus and the disease it causes. Thank heavens that international cooperation and data sharing is at an unprecedented high. It is clear that we have a lot more to learn about how the virus goes about its business and how our bodies react to it.
With each new insight come fresh ideas of how to protect ourselves. For now, I will give you just one. If one of the problems is that the virus down-regulates interferon production, why not supplement those infected with interferons we already use in the treatment of other diseases? Well, one of the two drugs added to a four-drug regimen—what I call the “Hong Kong Cocktail”—that reduces viral load and hastens the healing of mild to moderately ill Covid-19 patients is… an interferon!
We are in this for the long haul. The scientific journey is just getting underway. I am confident that we can beat this disease with a combination of enlightened public health policies, transparent rigorous clinical trials, and the power of our science.
Originally posted on Forbes (May 27, 2020)