Crimean-Congo Hemorrhagic Fever Virus Monoclonal Antibodies: A Work In Progress
(Posted on Thursday, December 1, 2022)
Crimean-Congo Hemorrhagic Fever is among the deadliest diseases in the world; a tick-born disease touting a mortality rate of up to 40%. First discovered almost eight decades ago, there is no effective treatment at present. Here we describe a work in progress of attempts to develop monoclonal antibodies to prevent and treat this deadly disease.
CCHFV Background
The virus was first identified in Crimea in 1944 and later in the then-Belgian Congo in 1967. More recent reports date the virus as far back as ancient Celtic settlements between 1500 and 1100 BCE. Since its discovery, outbreaks have plagued dozens of countries in Eastern Europe, the Middle East, Africa, and South Asia. The most substantial was an outbreak in Turkey from 2002 to 2008, in which 3,128 cases were reported.
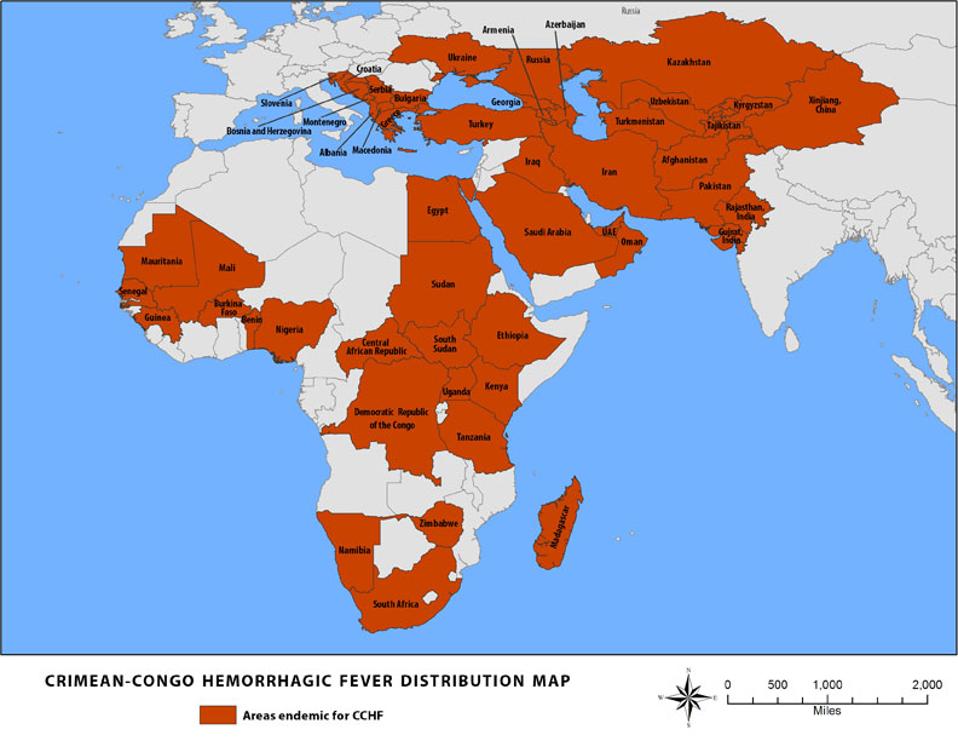
FIGURE 1: CCHFV distribution map.
CDC
CCHFV is most commonly transmitted by ticks. In southeast Iran, 31 different species of tick carry the pathogen. The pathogen has also been found in hares, hedgehogs, rats, birds, and domesticated animals such as sheep, goats, and cattle.
The virus is a member of the genus Orthonairovirus, family Nairoviridae of RNA viruses. Other viruses in this genus include the Dugbe virus, the Nairobi sheep disease virus, and the Kasokero virus. The figure below illustrates the basic structure of CCHFV.
FIGURE 2: CCHFV virus structure.
BENTE ET AL.
Throughout the decades, attempts have been made at vaccines and treatments for the virus and its subsequent disease, but all were tabled due to low efficacy or associated toxicity. A new antibody treatment could be helpful for hundreds impacted by the disease annually and thousands that could be spared from infection altogether.
CC5 Human Monoclonal Antibodies
The first objective for researchers Durie et al. was to find a worthwhile target for monoclonal antibody treatment. All previous treatments had failed, so a novel site would be a quality starting point.
Their strategy was to use a previously discovered antibody that prevented severe disease in mice against CCHFV, but failed to do so in humans. They analyzed the antibody, 13G8, and its primary binding site: CCHFV glycoprotein GP38.
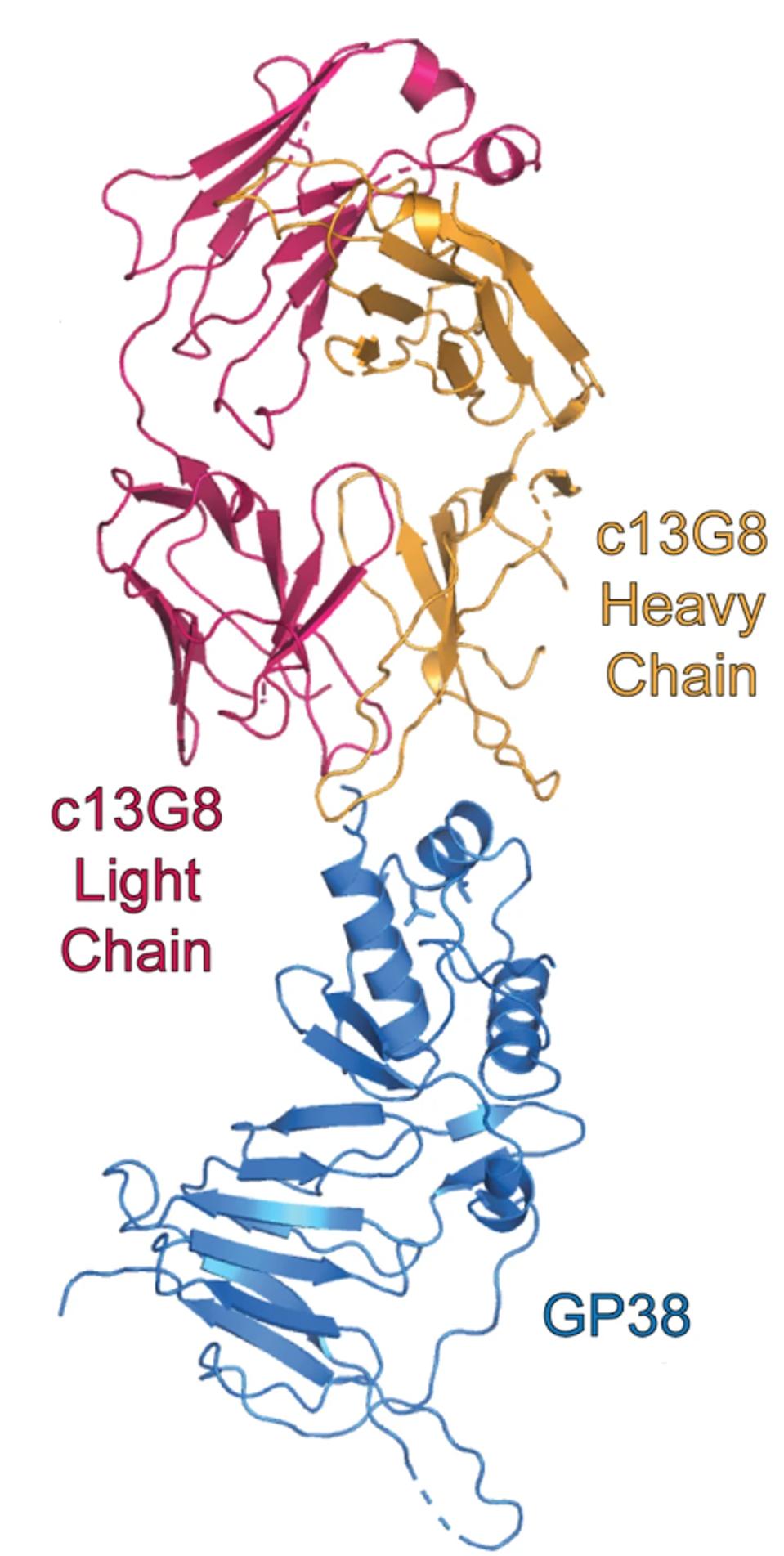
FIGURE 3: Mouse monoclonal antibody 13G8 binding GP38.
DURIE ET AL.
Using this site as a template, the researchers searched for human monoclonal antibodies targeting this same site. They pulled sera from six verified survivors of CCHFV infection and isolated a panel of antibodies for further testing from one patient: CC5.
Of the seven antibodies isolated from CC5, all matched or exceeded the binding affinity of the mouse antibody 13G8. The three most potent binders overall were CC5-6, CC5-16, and CC5-17. Three of the seven human monoclonal antibodies directly competed with the binding epitope of 13G8: CC5-6, CC5-12, and CC5-17. This overlap would suggest that GP38 is a prime target for antibody binding moving forward for CCHFV treatments.
Upon further cryo-electron microscopy experiments, Durie et al. found that CC5-17, one of the stronger binding human monoclonals, attacks GP38 at a differing angle of 22 degrees, which may account for the more thorough binding affinity.
FIGURE 4: Differing binding angles of the mouse antibody 13G8 and the human antibody CC5-17.
DURIE ET AL.
The researchers also note the binding capability of CC5-17 to another closely related virus, the Aigai Virus. The GP38 binding site is shared between the two viruses, and minor mutational differences do not affect binding affinity.
Unfortunately and unsurprisingly, the human antibodies were again non-neutralizing in human in vitro models. The antibody may still protect against severe disease and death in human CCHFV patients, but it seems the GP38 antibody binding site is exclusively nonbinding. This suggests another receptor could yield a more substantial neutralizing capacity when bound by an as-of-yet-undiscovered antibody. The CC5 antibodies may have some diagnostic value but fall short as a tool for protection.
Concluding Thoughts
While it is disappointing that CC5-17 and its CC5 alternatives were not innately neutralizing, their shortcoming could enable another treatment’s success in the future. The GP38 binding site is a prime target for broad binding affinity. GP38 likely has a significant role in virus maturation and localization of viral particles to the host cell’s surface. A viable antibody candidate could disrupt this process and effectively neutralize the virus. GP38 could also serve as a starting platform for vaccine treatment soon. Otherwise, different binding sites should be investigated throughout the virus genome in the search for a treatment.
This study confirms the possibility of highly effective anti-CCHFV treatments; we need only find them.