Despite Conflicting Evidence, FDA Approves Covid-19 Drug Remdesivir
(Posted on Monday, October 26, 2020)
One vial of the drug Remdesivir lies during a press conference about the start of a study with the Ebola drug Remdesivir in particularly severely ill patients at the University Hospital Eppendorf (UKE) in Hamburg, northern Germany on April 8, 2020,
POOL/AFP VIA GETTY IMAGES
On Friday, the U.S. Food and Drug Administration (FDA) gave its first official stamp of approval to a Covid-19 drug — remdesivir. The repurposed antiviral, now also known by the generic name of Veklury, was granted emergency use authorization (EUA) in May and has been administered intravenously to hospitalized Covid-19 patients ever since.
The conclusion of a study sponsored by the National Institutes of Health (NIH), which published its final report two weeks ago, appears to be the main reason regulators moved forward with approval. But like the preliminary research that paved the way for the drug’s EUA, the NIH study shows that remdesivir was at best only moderately effective in reducing the suffering and death of those who took it. Not too shabby, but not particularly heartening either.
Just last week, newly published interim results from the largest clinical study of remdesivir (and three other drugs) to date, the World Health Organization’s (WHO) Solidarity trial, cast some doubt on even these modest benefits. Involving more than 11,300 people across 30 countries, the study found that Covid-19 patients prescribed remdesivir weren’t any likelier to survive than those who weren’t. Neither did they have a shorter or less painful hospital stay.
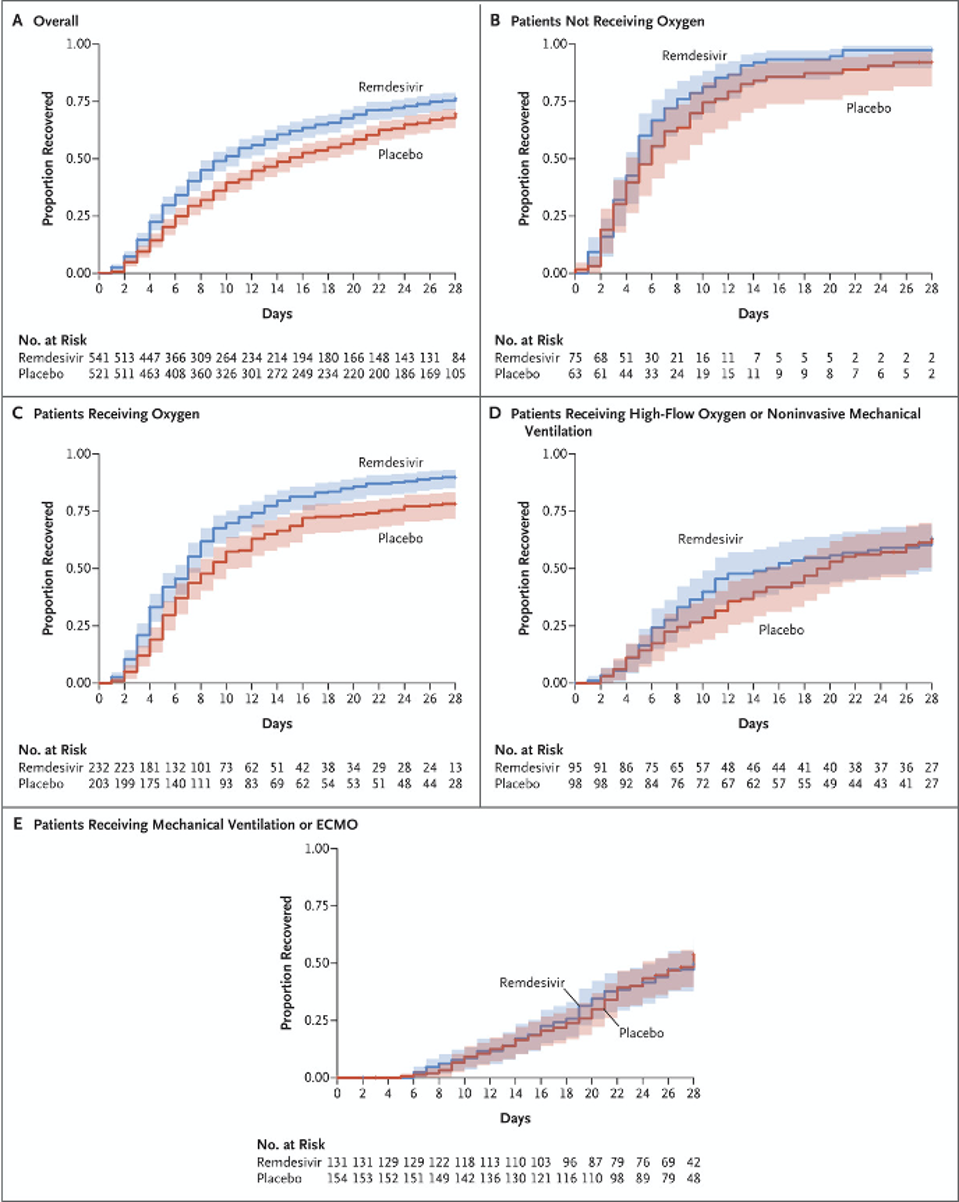
Charts from the final NIH report show the moderate differences in the health of Covid-19 patients receiving remdesivir and those receiving a placebo.
JH BEIGEL ET AL. N ENGL J MED 2020. DOI: 10.1056/NEJMOA2007764
Gilead, the pharmaceutical giant behind remdesivir, argued in a company statement that the findings of the WHO trial are too broad to be conclusive. By the same token, however, it could also be said that the other remdesivir studies are too small. While the NIH study — unlike the Solidarity trial — did involve a placebo group, it also only administered the drug to few more than 500 patients. It can hardly be assumed that the outcomes of this very specific patient population are more representative than those of several thousand people around the world.
A safer and more reasonable conclusion, given the data at hand, is that remdesivir may be useful for some, but not all. In this respect the drug is hardly unique; rare is it that one therapeutic regimen fits all. But for a drug so patchy and uncertain in its efficacy, it sure is expensive. An average five-day treatment course costs $2,340 for patients covered by government health plans and $3,120 for those privately insured. What’s more, Royal Bank of Canada analysts predict that Gilead will make about $2.3 billion in revenue off remdesivir sales in 2020 alone — quite a pretty penny for a marginally effective product.
Further investigation is needed to confirm the real extent of remdesivir’s positive effects. Let’s hope until then the next Covid-19 drug the FDA approves has a less conflicting and more robust body of evidence behind it. Otherwise, we might end up with an assortment of treatments that do more financial harm to patients than clinical good.
Gilead has been contacted for comment.
Originally published on Forbes (October 26, 2020)