Hope For A New Treatment On The Horizon For Zika Virus
(Posted on Wednesday, November 23, 2022)
Researchers from Duke University, UC Berkeley, Purdue University, and elsewhere have made what will likely be a significant breakthrough in drug development to treat Zika Virus infections. They discovered an unusual monoclonal antibody, not of the common immunoglobulin G family, but rather immunoglobulin M. This antibody is extraordinarily potent in neutralizing the Zika Virus in tissue cultures, as well as in live animal experiments.
Monoclonal antibodies are among our greatest assets in treating and preventing virus-induced disease. While the spotlight has focused squarely on Covid-19 monoclonal antibodies throughout the pandemic, antibody candidates for other severe pathogens have also progressed. Here we describe a new antibody candidate that neutralizes the Zika Virus, which is responsible for thousands of infections annually.
While not nearly as prevalent as its peak in 2016, documented Zika Virus infections still occur in over 80 countries, with roughly 18,000 cases per year. Infections most often occur in areas closer to the equator, as mosquitoes are the primary mode of transmission for the virus.
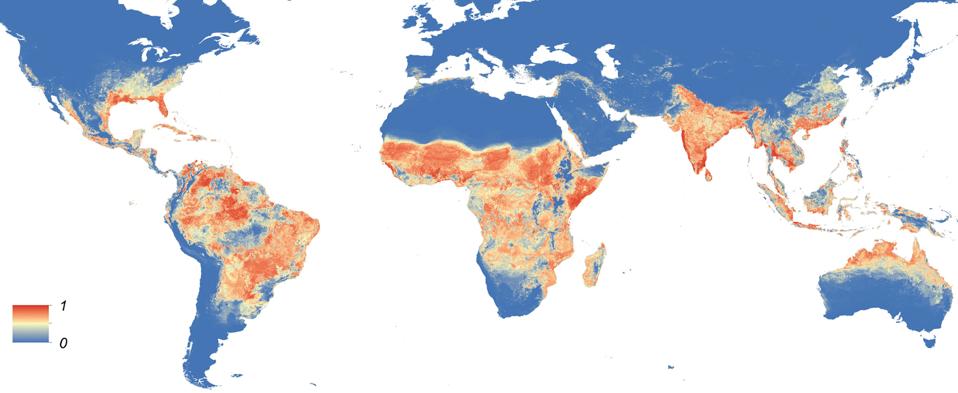
The incubation period for Zika Virus disease is between three and 14 days, at which point symptomatic patients yield fever, rash, conjunctivitis, joint pain, and headache for up to a week. One of the more severe Zika Virus complications occurs in pregnant hosts. Roughly 14% of host fetuses develop severe brain and eye defects. Many cases result in stillbirth, premature birth, or miscarriage.
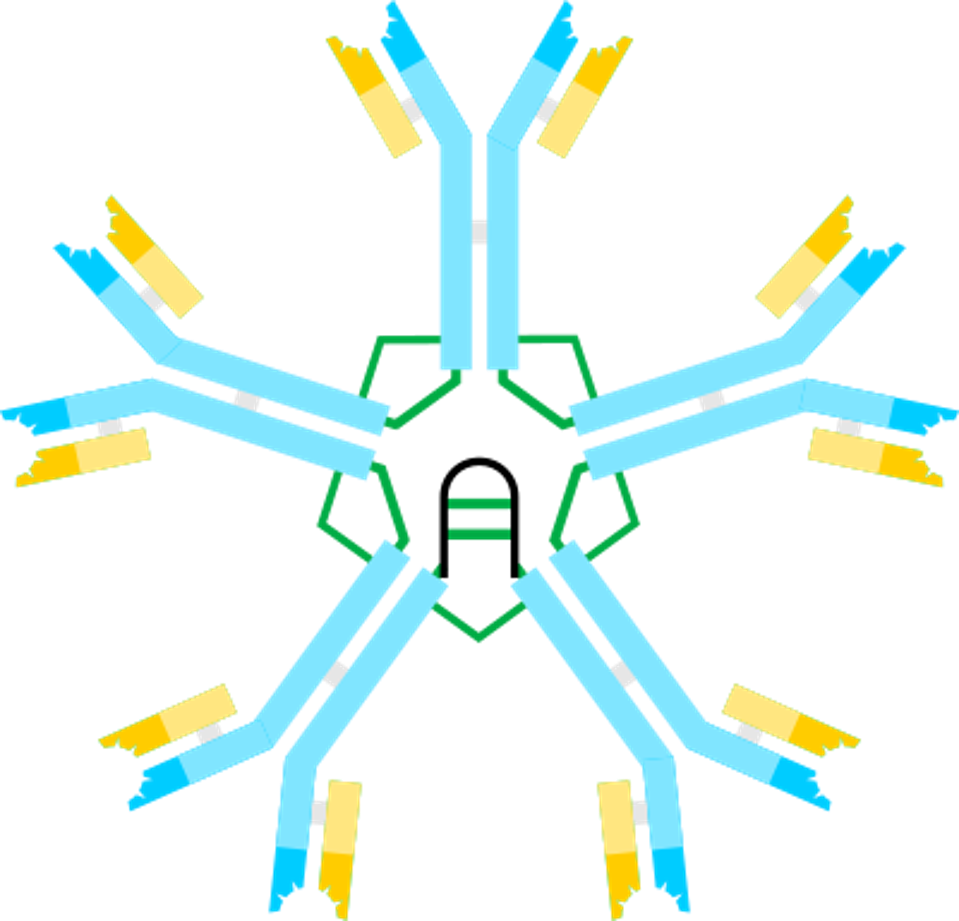
Researchers Singh et al. aimed to discover a monoclonal antibody treatment to placate the virus that still rages in tropical regions. The scientists were surprised to find that a specific type of antibody, immunoglobulin M (IgM), was particularly active concerning Zika Virus immunity for the fetus during pregnancy. The vast majority of antibodies are immunoglobulin G (IgG). While most antibodies are a single ‘Y’ shaped monomer, the IgM antibody presents in a set of five, or a pentamer. IgM antibodies are the largest produced antibody and are the first to respond to initial exposure to an antigen. The monomers are bound to their adjacent monomer by a disulfide bond, and a joining chain keeps the large antibody intact.
ARTUR JAN FIJALKOWSKI
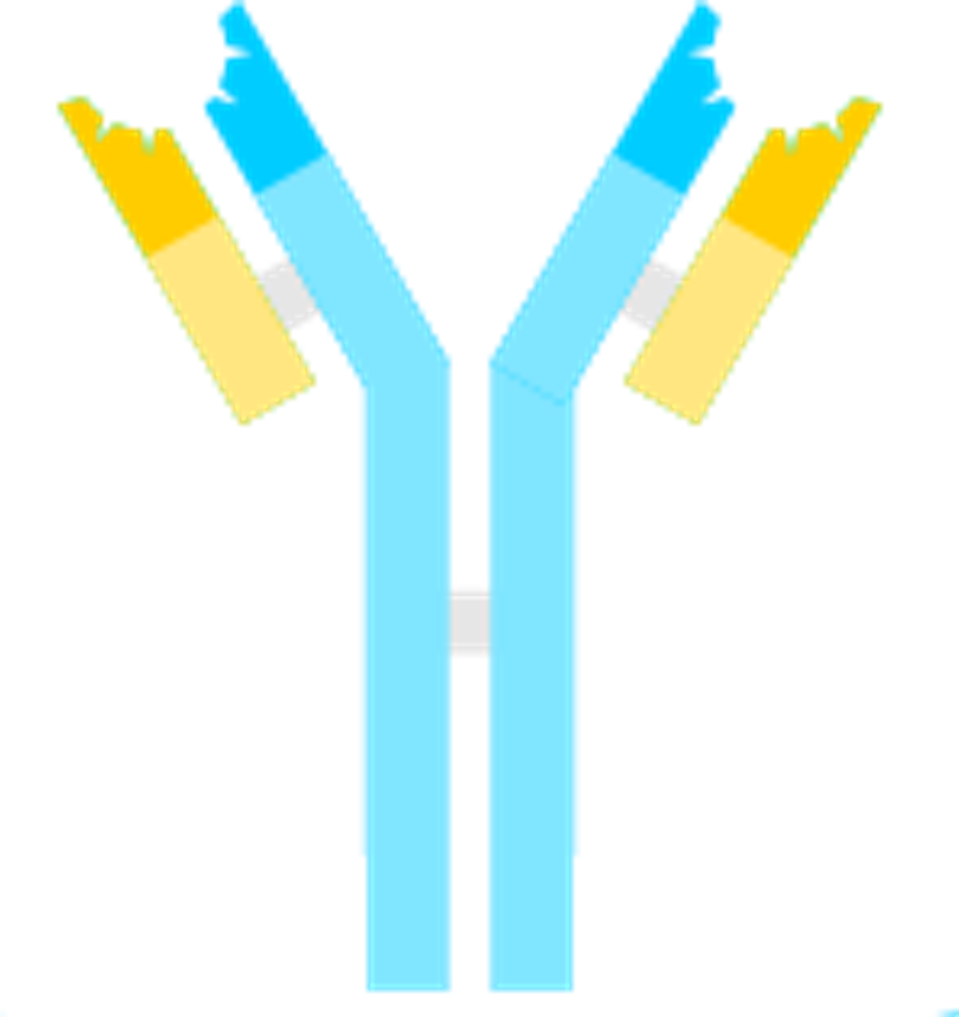
FIGURE 2: Immunoglobulin M antibody type (top) and immunoglobulin G antibody type (bottom). Disulfide bonds are depicted in green and the joining chain is depicted in black.
ARTUR JAN FIJALKOWSKI
Singh et al. extracted plasma IgM to test for binding and neutralization from a cohort of 10 pregnant Brazilian women during the 2015-2016 Zika Virus outbreak. They eventually isolated one Zika Virus monoclonal antibody candidate, DH1017.IgM, which demonstrated the most robust Zika-neutralizing capacity in early testing.
In addition to neutralizing the parental Zika Virus, DH1017.IgM also neutralized ZIKV PRVABC59 and ZIKV H/PF/2013, two prominent variants of the parental virus. DH1017.IgM also had a lower mutation rate than other antibody candidates in early testing, meaning neutralization capacity is unlikely to change due to chance mutations in the antibody.
Notably, Singh et al. found that the pentameric form of the IgM is crucial to DH1017 binding. As a Fab fragment, meaning the arms of the ‘Y’ shape solely, the antibody bound poorly. DH1017 bound roughly 20-fold stronger as an IgG monomer, but as an IgM pentamer, the antibody bound more than five-fold stronger than the monomer. In parallel, neutralization for IgM was 40-fold stronger than the IgG monomer.
The researchers conducted further tests on mice models to determine in vitro neutralization for DH1017. They found that the IgM antibody protects against severe and lethal cases of Zika Virus infection at lower 50 or 100 microgram doses but protects against viremia much more efficiently at higher doses. Again, the researchers found that the IgG monomer version of DH1017 fails to achieve the marks set by the pentameric IgM.

FIGURE 3: Neutralizing potency of (A) the DH1017 antibody fragment, (B) the DH1017 IgG antibody, and (C) the DH1017 IgM antibody.
SINGH ET AL.
Singh et al. note that the five-armed IgM antibody “may contact up to five epitope pairs compared to a single epitope pair for the bivalent DH1017.IgG.” This would explain the vastly increased binding and neutralization. Picture a chest with a single lock and a chest with five locks. Which is more secure?
The researchers also found that the IgM may bind epitope pairs across different virus particles, creating a cross-linked virion epitope. Now picture multiple chests chained and locked together. The IgM antibody form presents fascinating advantages over its IgG counterpart.
The DH1017 antibody, like many antibodies treating viruses primarily impacting lower-income nations, would need to be produced at a low cost to ensure those who need the treatment most could afford it. Current technologies allow monoclonal antibodies to be produced at $200 and $250 per gram. DH1017 could be a godsend for those still impacted by Zika Virus, particularly pregnant women in low-income countries.
In a broader sense, this study opens a new avenue for monoclonal antibody development. It is clear that pentameric IgM antibodies bind and neutralize much more effectively than monomeric IgG antibodies. It would be relatively straightforward to convert potent IgG antibodies to IgM by replacing the Fc portion of the antibody. If researchers could harness that advantage and engineer antibodies to other global antigens, such as SARS-CoV-2, the reward would be well worth the effort.
Read the full article on Forbes.