Hope For Universal, Ready-Made CAR T Therapy For Multiple Myeloma
(Posted on Tuesday, February 28, 2023)
Micrograph of myeloma tumor from bone marrow biopsy
GETTY
A hallmark characteristic of CAR T therapy is that it is individually crafted. Each patient receives a unique cancer-fighting infusion made from their own immune cells. The crucial process of genetically modifying the cells, however, is lengthy and costly. Early study results from the UNIVERSAL clinical trial test a promising solution: a ready-made version of CAR T therapy for multiple myeloma. The treatment shows encouraging safety and efficacy profiles, bringing the concept of off-the-shelf CAR T therapy one step closer to clinical translation.
Multiple Myeloma and CAR T Therapy
Multiple myeloma (MM) is a cancer of the plasma cells in the bone marrow. This cancer initially responds well to chemotherapy, targeted therapy and other first line therapeutics. Unfortunately, the disease often returns and grows resistant to prior treatments. When other options are no longer effective, CAR T cells can achieve a durable response.
There are currently two FDA-approved CAR T cell products for multiple myeloma: Ide-cel and cilta-cel. Ide-cel impressively decreases signs of multiple myeloma in 72% of patients, and cilta-cel 98% of patients. Both require the extraction and modification of patient cells to target B cell maturation antigen (BCMA), a biological tag found on the surface of mature plasma cells. Note that while this antigen is a common target for anti-myeloma treatments, research is underway to identify more potential targets.
Shortcomings of CAR T Therapy
The process of extracting, genetically modifying, proliferating and infusing cells for each patient is resource-intensive and expensive, with a delay of one month or more. A majority of patients also require bridging therapy—treatments to control their disease during the wait. For this reason only a minority of patients proceed with CAR T.
One possible solution is to have pre-prepared CAR T cells that many patients could use instead of cells tailored to a single individual. Many have described this as “off-the-shelf.” Researchers believe that a ready-made CAR T product could be administered within days and reduce costs associated with manufacturing and bridging therapy. Here, we describe clinical trial results which use already prepared CAR T cells to treat patients with multiple myeloma.
Off-the-Shelf CAR T Cells
To make off-the-shelf CAR T cells, Mailankody et al. took T cells from three healthy donors and engineered them with new chimeric antigen receptors (CARs) in place of a patient’s cells. The risk with this method is that the body may reject the donor cells, as often occurs with tissue transplantation. In consequence, the researchers’ CAR T design and study protocol address major hurdles such as graft-vs-host disease (GvHD) and CAR T cell rejection.
The researchers employed three safety features to reduce tissue rejection. First, they modified a familiar CAR T cell design used for myeloma. Typical components include an anti-BCMA antibody fragment to detect the plasma cells; a 4-1BB costimulatory molecule to support the cell’s survival and proliferation; and a CD3γ signaling domain to release cancer-killing chemicals. The notable new addition is the off-switch illustrated in Figure 1. This off-switch allows the team to deactivate the CAR T cells if needed by implementing a monoclonal antibody called rituximab.
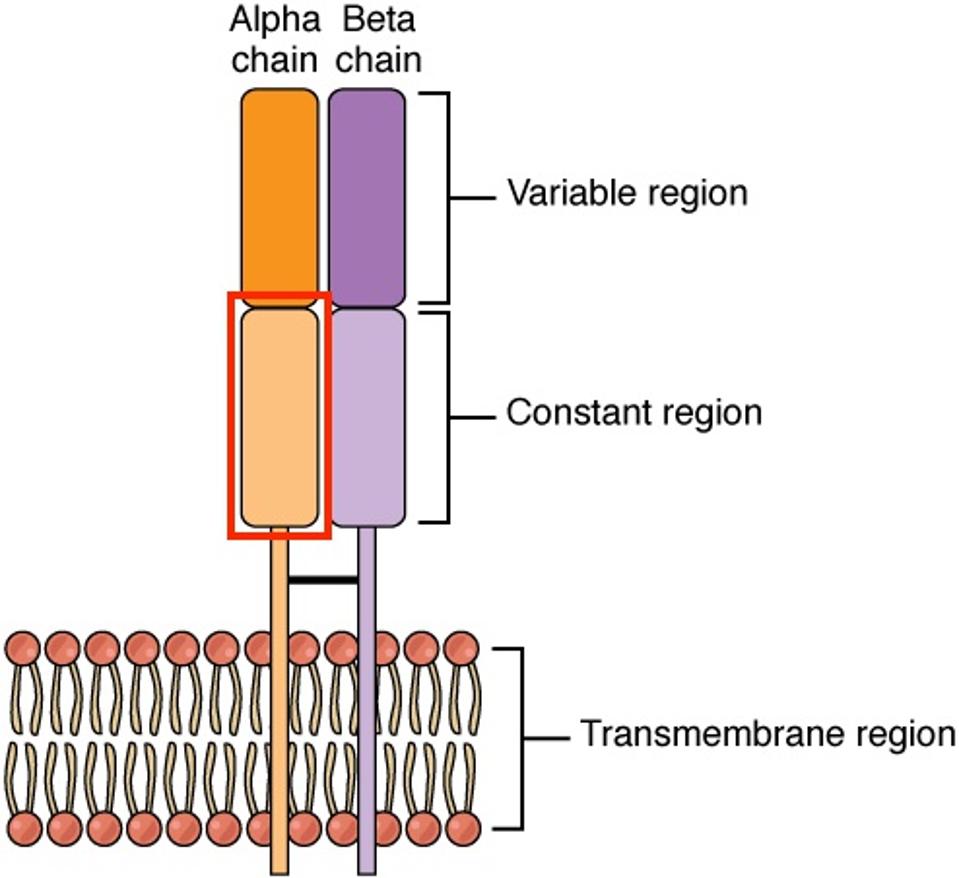
Next, the team stopped the expression of a gene called T cell receptor alpha constant (TRAC) in the CAR T cells. They used gene-editing enzymes called Transcription Activator-Like Effector Nucleases—TALENs for short—which can recognize and cut out this gene associated with graft-vs-host-disease. The knockout reduces the expression of T cell receptor complexes (Figure 2) on the CAR T cell surface. With fewer receptors to communicate with, host T cells are less likely to recognize the CAR T cells and eliminate them.
Lastly, the researchers altered common preparation procedures. Patients usually begin with an immunosuppressive therapy called lymphodepletion prior to CAR T cell infusion. In addition to this standard practice, the team incorporated a new monoclonal antibody to remove threats before they turned into enemies. The monoclonal antibody targets host immune cells with glycoprotein CD52 on their surface. These host cells can mediate graft-vs-host disease and would counter the CAR T infusion if left alone. With this looming threat eliminated, the CAR T cells can proliferate freely.
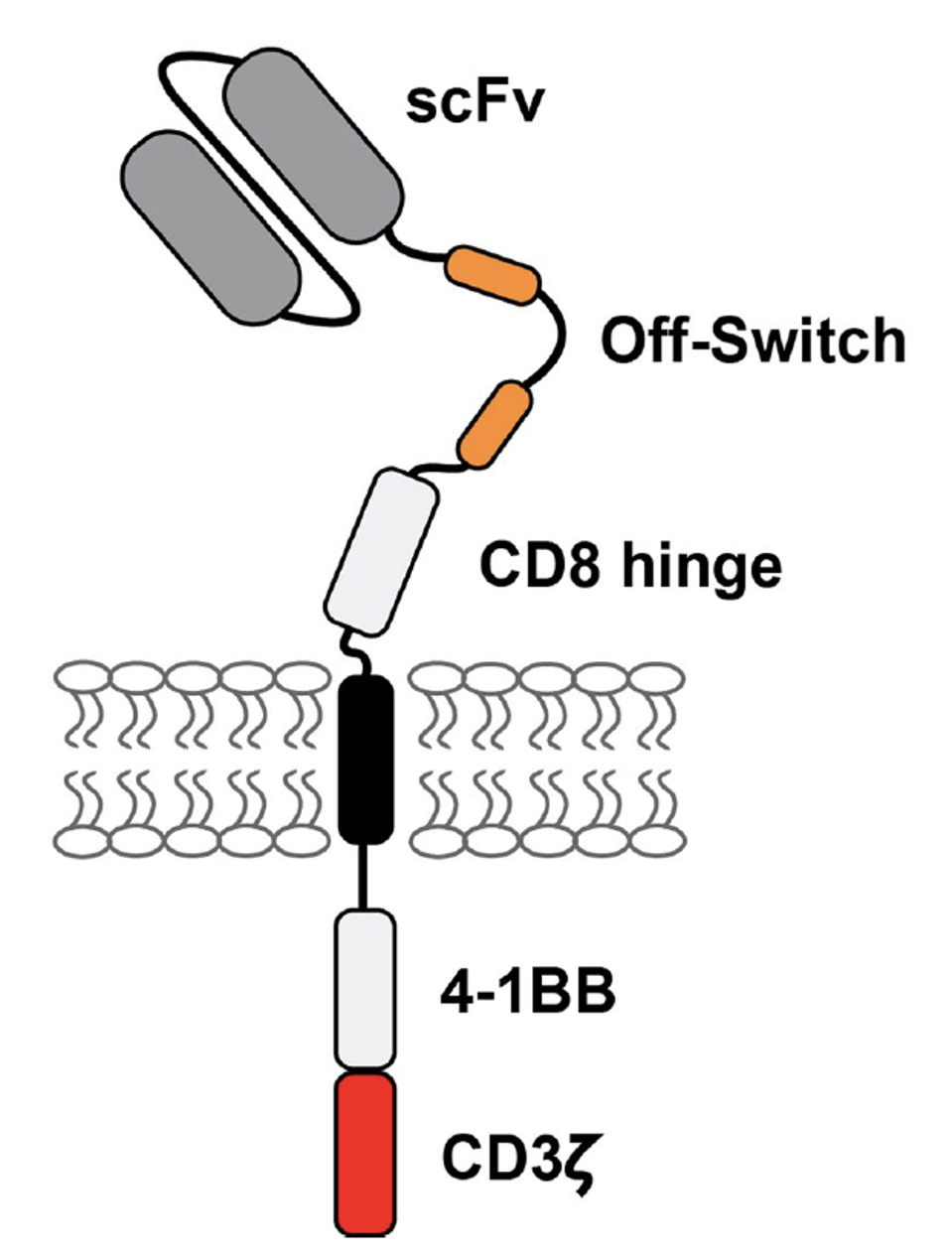
FIGURE 1: This study’s second generation CAR T design features familiar components: anti-BCMA single chain variable fragment (scFV), a CD8 hinge, a 4-1BB costimulatory molecule and a CD3γ signaling domain. New to the design is the unique addition of two peptides. These proteins allow the CAR T cell to essentially turn off in the presence of an anti-CD20 monoclonal antibody called rituximab.
MAILANKODY ET AL., 2023.
FIGURE 2: Structure of a T cell receptor alpha-beta (TCR αβ) complex. The alpha and beta chains allow T cells to recognize antigens presented by major histocompatibility complexes (MHC). The presence of this structure is also associated with graft-vs-host disease. The researchers knocked out the gene which encodes for the constant region of the alpha chain (TRAC, highlighted in red) to prevent CAR T cell rejection.
WIKIPEDIA
Encouraging Safety and Tolerability Results
A total of 43 patients were given a lymphodepletion regime and escalating doses of the experimental CAR T cell infusion (see Figure 3). These patients failed at least three prior lines of treatment—none with prior exposure to BCMA-directed CAR T therapy. Lymphodepletion was given up to five days before infusion. The patients did not need treatment to manage their cancer between enrollment and CAR T cell infusion.
Adverse Events
The trial produced promising safety profile results. None of the patients experienced graft-vs-host disease. The authors attribute this success to the knockout of the T cell receptor gene knockout (TRAC). This does not mean, however, that there were no negative responses to the therapy. All 43 patients experienced some adverse event, but none were sufficient to terminate the trial.
The most common CAR T side effects are cytokine release syndrome and neurotoxicity. Both conditions are caused by the abundant release of immune chemicals, and the severity of the side effects increases on a scale of one to four, with one representing mild symptoms to four at life threatening. Half of patients experienced mild to moderate cytokine release syndrome and 14% percent experienced mild to moderate neurotoxicity. Only one person experienced Grade 3 or higher neurotoxicity, which is a slightly lower but notable difference from rates of other anti-myeloma CAR T therapies.
The use of a new monoclonal antibody during the lymphodepletion regime did not seem to increase the number of severe infections compared to other custom-made myeloma CAR T therapies. Notwithstanding, the authors do recommend viral monitoring of cytomegalovirus (CMV), a common virus that many carry but does not impact healthy people. The monoclonal antibody increases the risk of reactivating this virus, and the infection will have to be treated with prophylaxis.
Response Rates
The CAR T therapy was well-tolerated. The trial yielded an overall response rate of 56%. The group who received a 320 million CAR T cell dose had a promising 71% response rate with a median duration of response of 8.3 months. As illustrated in Figure 4, six people in this cohort had undetectable signs of their multiple myeloma.
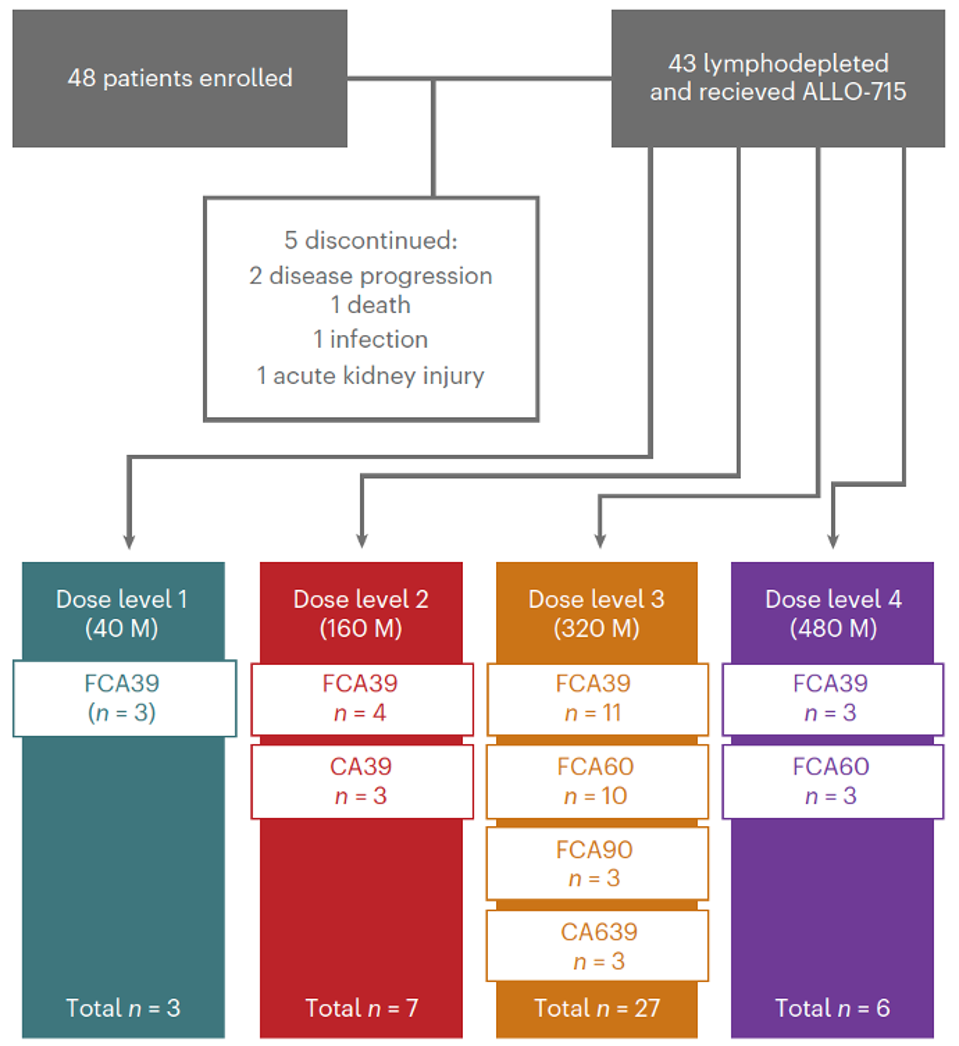
FIGURE 3: Flow diagram of subject enrollment. A total of 43 people received the treatment. Abbreviations: M, 106 CAR T cells dose. Lymphodepletion prior to treatment: C, cyclophosphamide; F, fludarabine; A39/60/A90, different doses of anti-CD52 monoclonal antibody.
MAILANKODY ET AL., 2023.
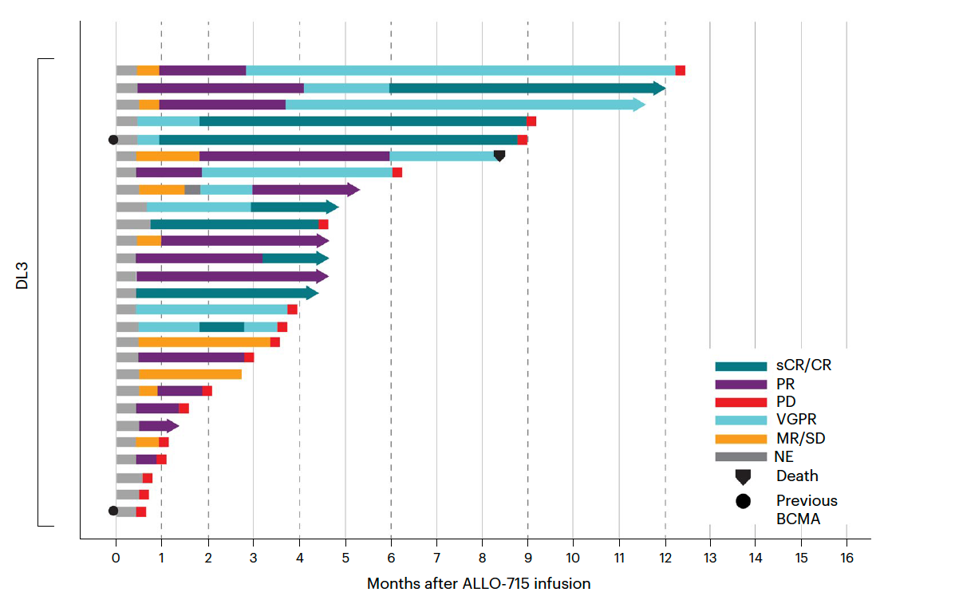
FIGURE 4: Duration of response for patients who received a 320 million CAR T cell dose (also referred to as DL3, 3rd level dose) with lymphodepletion regime of fludarabine, cyclophosphamide and varying doses of the monoclonal antibody (FCA). Abbreviations: NE, not estimable; MR/SD, minimal response/stable disease; VGPR, very good partial response; PD, progressive disease; PR, partial response; sCR/CR, stringent complete response.
MAILANKODY ET AL., 2023.
Looking Forward
A universal, ready-made CAR T therapy may not be a distant dream. The early results from the UNIVERSAL trial demonstrate that off-the-shelf CAR T cells can perform safely if the cell design and lymphodepleting regime accurately address possible donor rejection. These cells pose one possible solution to reducing the labor, manufacture and time costs associated with already-established CAR T therapy for multiple myeloma.
In spite of this, off-the-shelf CAR T therapy may still be vastly prohibitive to most who need it. This underlying problem of access may require other improved technologies such as the use of mRNA to modify cells in vivo, as well.