How One Covid-19 Patient’s Infection Foreshadowed The Rise Of New Variants
(Posted on Thursday, February 18, 2021)
Random variation is an essential component of all living things. It drives diversity, and it is why there are so many different species. Viruses are no exception. Most viruses are experts at changing genomes to adapt to their environment. We now have evidence that the virus that causes Covid, SARS-CoV-2, not only changes, but changes in ways that are significant. This is the fifteenth part of a series of articles on how the virus changes and what that means for humanity. Read the rest: part one, part two, part three, part four, part five, part six, part seven, part eight, part nine, part ten, part eleven, part twelve, part thirteen, and part fourteen.
Colorized scanning electron micrograph of an apoptotic cell (pink) heavily infected with SARS-CoV-2 virus particles (green), isolated from a patient sample. Image captured at the NIAID Integrated Research Facility (IRF) in Fort Detrick, Maryland.
BSIP/UNIVERSAL IMAGES GROUP VIA GETTY IMAGES
Let’s recap my last few articles on parallel evolution, the term researchers have conceived to describe the ability some viruses have—namely, coronaviruses and influenza—to evolve independently of one another across multiple scales of time and space. Last week I discussed the origins of this theory in influenza research, the recent studies that apply it to SARS-CoV-2, and the London patient, the first of many immunocompromised and persistently infected Covid-19 patients with documented evidence of parallel mutations. Next in line was the Boston patient, followed shortly thereafter by the Pittsburgh patient. Through the lens of these case studies I examined the myriad aspects and potential consequences of viral variation—where it occurs, why it occurs, and what it means for the future of the pandemic.
Today I’ll turn my focus to yet another case study involving parallel mutations, that of the Italian patient, which was published in The Lancet last month. Unlike the examples we’ve discussed thus far, the research paper doesn’t disclose whether or not this patient, a 59-year-old man, had underlying health conditions prior to his Covid-19 diagnosis. He did, however, experience a similarly persistent infection that evidently lasted a few months. Though the details regarding when the Italian patient’s disease course began and ended, as well as the treatments he received, if any, also aren’t elaborated, researchers were able to extract and sequence viral samples at least twice, first in August and again in September. They then compared these samples with isolates of SARS-CoV-2 strains that were circulating in Italy either concurrently or earlier on in the pandemic.
One of the more striking implications of the theory of parallel evolution is that some of the mutations that develop in persistently infected Covid-19 patients either appear in or foreshadow new variants that emerge and become dominant in the wider population. Excluding D614G, the mutation that reached near universal prevalence among active strains last spring, the viral sample taken from the Italian patient in August had a total of nine mutations that distinguished it from early strains (Table 1). Three were located in the ORF1a region of the virus (D1639N, P4715L, and A6914V), two in the receptor binding domain of the spike protein (Q493K and N501T), two in the N-terminal domain of the spike protein (K182N and L242 del), and two in the N protein (R203K and G204R).
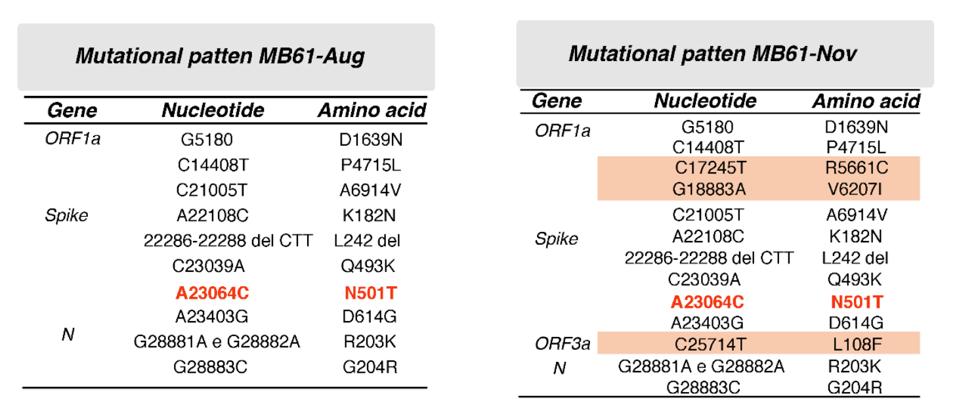
Table 1. Mutational pattern of the two isolates MB61-Aug and MB61-Nov, which were obtained from the same patient 4 months apart.
SOURCE: “FIRST DETECTION OF SARS-COV-2 SPIKE PROTEIN N501 MUTATION IN ITALY IN AUGUST, 2020” HTTPS://WWW.THELANCET.COM/JOURNALS/LANINF/ARTICLE/PIIS1473-3099(21)00007-4/FULLTEXT#SUPPLEMENTARYMATERIALOf these nine, the easiest to make inferences about are in the receptor binding domain, a key target for Covid-19 drugs and vaccines (Figure 1). The most recognizable is N501T, an amino acid change that occurred in the same position as N501Y, one of the defining characteristics of the B.1.1.7 (UK), B.1.351 (South Africa), and P.1 (Brazil) variants. It also appeared independently in the viral genomes of the Boston and London patients. Thanks to laboratory experiments, we now know that N501Y—shorthand for the substitution of the polar, uncharged asparagine with tyrosine, a larger nonpolar molecule—increases the affinity of the virus, though most likely with only minor changes to immunogenicity. N501T, which sees asparagine swapped for threonine, is far less dramatic, but still notable for its symmetry. While another spike protein mutation, Q493K, has yet to make an appearance in a globally prevalent variant, it marks another point of convergence between the Italian and Boston patients—a parallel that warrants further investigation. Not for nothing, the same in-lab studies that confirmed the increased affinity of N501Y found that another mutation at the 493 site, Q493H, had a comparable effect.
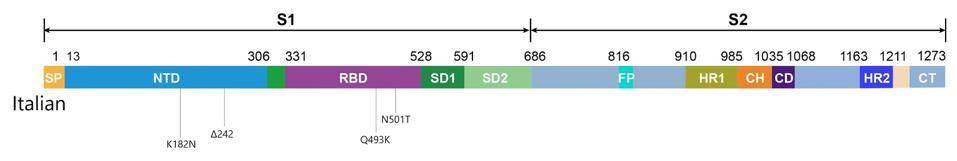
Figure 1. Linear visual representation of spike protein in viral genomes from the Italian patient.
AUTHOR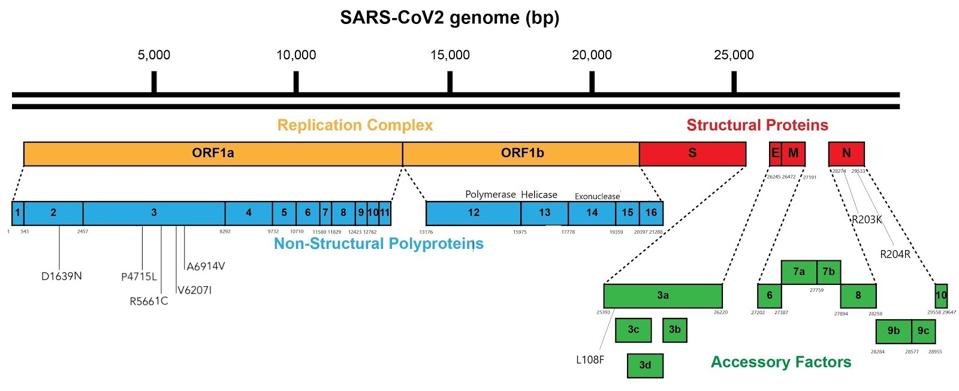
Figure 2. Linear visual representation of the mutations outside the spike protein in viral genomes from the Italian patient.
AUTHORIn my story on the Pittsburgh patient, I argued that the N-terminal domain is one to watch due to the significant effect mutations in the region have on the ability of SARS-CoV-2 to evade our immune defenses, whether naturally produced or mediated by preventive medicine. The N-terminal domain mutations of the Italian patient (K182N and L242 del), though not identical to any we’ve seen thus far, do fall within close range of the deletions at the 183 and 242 sites that occurred in the Pittsburgh patient, as well as a Q183H substitution noted in the Boston patient. The B.1.351 (South Africa) variant also has a deletion in the 242 position.
Moving on, the second sample collected from the Italian patient in November had three new mutations in addition to the original nine, two of which were located in the ORF1a region (R5661C and V6207I) and one in ORF3a (L108F). Though the significance of mutations in these areas isn’t widely understood, like the N-terminal domain the ORFs may have a role to play in modulating the efficacy of SARS-CoV-2 that will become either more discernible or more deleterious down the line.* All the more reason to keep a close eye on both.
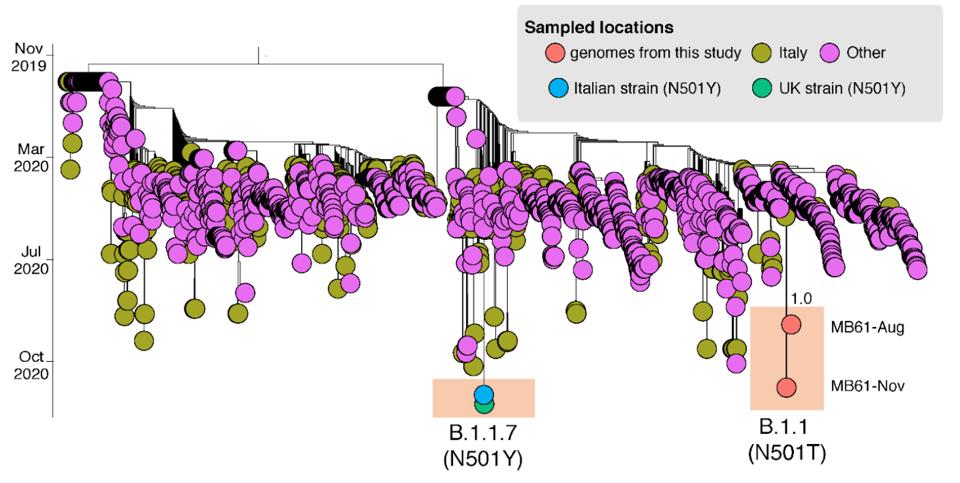
Figure 3. Time-scaled maximum likelihood tree including MB61-Aug and MB61-Nov plus 2137 sequences representative of the global SARS-CoV-2 epidemic.
SOURCE: “FIRST DETECTION OF SARS-COV-2 SPIKE PROTEIN N501 MUTATION IN ITALY IN AUGUST, 2020”While the case study of the Italian patient doesn’t substantiate the theory of parallel evolution as neatly as, say, the London or Boston patients, the parallels become clearer when we take into account local transmission in his country of origin (Figure 3). Italian researchers estimate that by the time they analyzed the first sample from the patient in August, variants with alterations to the 501 site were already circulating in northern Italy. A lack of genomic surveillance, not just in Italy but worldwide, helped these variants remain under the radar—until September, that is, when reports of the B.1.1.7 variant and its signature N501Y mutation first emerged. If their predictions are true, this means the Italian patient did in fact foreshadow the arrival of more infectious strains. To say so may be too little too late, but not if we learn from this lesson going forward.
It is not only possible, but highly likely that Covid-19 will become a seasonal phenomenon, just like influenza and its cold-causing coronavirus cousins. In light of this, it becomes the task of scientists everywhere, myself included, to analyze the clinical and epidemiological data we currently have in our possession and make interpretations that will guide us in the future. The evidence that SARS-CoV-2 is a wily, capable, and ruthlessly adaptable adversary is piling up steadily. My next contribution to this body of evidence, and this series, will be an examination of the 677 mutations that are appearing in Covid-19 variants across the United States.
*

Table 2. Nonstructural proteins of coronaviruses and their functions.
SOURCE: “STRUCTURAL AND FUNCTIONAL INSIGHTS INTO NON-STRUCTURAL PROTEINS OF CORONAVIRUSES” HTTPS://PUBMED.NCBI.NLM.NIH.GOV/33242646/
Originally published on Forbes (February 18, 2021)