Molnupiravir: A New Hope For Prevention And Treatment Of Covid-19 And Other Dangerous Viruses
(Posted on Tuesday, March 16, 2021)

DANVILLE, PENNSYLVANIA, UNITED STATES – 2021/03/04: A signage seen outside Merck Cherokee Plant in Riverside, Pennsylvania as United States President, Joe Biden announced a partnership between Merck and Johnson & Johnson to produce more of the J&J
SOPA IMAGES/LIGHTROCKET VIA GETTY IMAGES
A new Covid-19 therapy has completed its phase two human trial and the results are promising. Molnupiravir, developed by Ridgeback Biotherapeutics LP and Merck & Co., reached its endpoint objective of reducing the length of Covid-19 infections, according to a Merck press release. This endpoint, among others, will be examined in greater depth upon the full data release from the trial.
Respiratory viruses like influenza, respiratory syncytial virus, and now Covid-19 are uniquely challenging to treat once symptoms arise. Because infections and symptoms for respiratory viruses are typically short-lived, the immune system quickly engages in viral replication suppression. Common form antivirals like Tamiflu and Xofluza for the flu, and now Remdesivir and monoclonal antibodies for Covid-19 must be administered within the first few days of infection to reduce symptom severity and infection duration.
For respiratory antivirals, their ideal use is for those recently exposed to viruses from family members or workplaces. Following known exposure, Influenza A antiviral Xofluza reduced transmission by 80% in a close-quarter family context. For Covid-19, monoclonal antibodies have been shown to achieve similar close-quarter transmission effectiveness, but they have a catch. Unlike Xofluza, monoclonal antibodies require infusion outside the home. Xofluza and antivirals like it are pill-based, capable of home-use, and can be ingested quickly after exposure. This new drug, Molnupiravir, may be a Xofluza-like alternative for Covid-19, which could be a powerful addition to our armamentarium, in addition to vaccines, especially for variants that seem to escape vaccines.
Molnupiravir was first developed as preventative medicine and treatment for SARS-CoV and MERS in the early 2000s. The drug has been previously shown to work against many viruses that employ an RNA-dependent RNA polymerase, which SARS-CoV-2 also has. The polymerase is the enzyme that copies the genetic material of the virus into new genetic material and produces the messenger RNAs that direct the production of all the viral proteins. Molnupiravir is a shape-shifter, called a tautomer. It assumes two forms, one which closely resembles uracil and the other cytosine.
Because it appears in these two different forms, once it is recopied, the replicating polymerase develops transition mutations, where a U nucleotide is converted to a C and a C to U. Copying RNA that contains Molnupiravir results in fatal flaws in the sequence, stopping replication, shortening infection, and limiting transmission. The figure below shows the replication process, and the red box indicates the polymerase mechanism with which Molnupiravir incorporates. The difference between the structure of an authentic nucleotide and Molnupiravir is apparently too subtle to trigger removal by the exonuclease repair function of the viral polymerase, a function that has bedeviled these of many other nucleoside inhibitors.
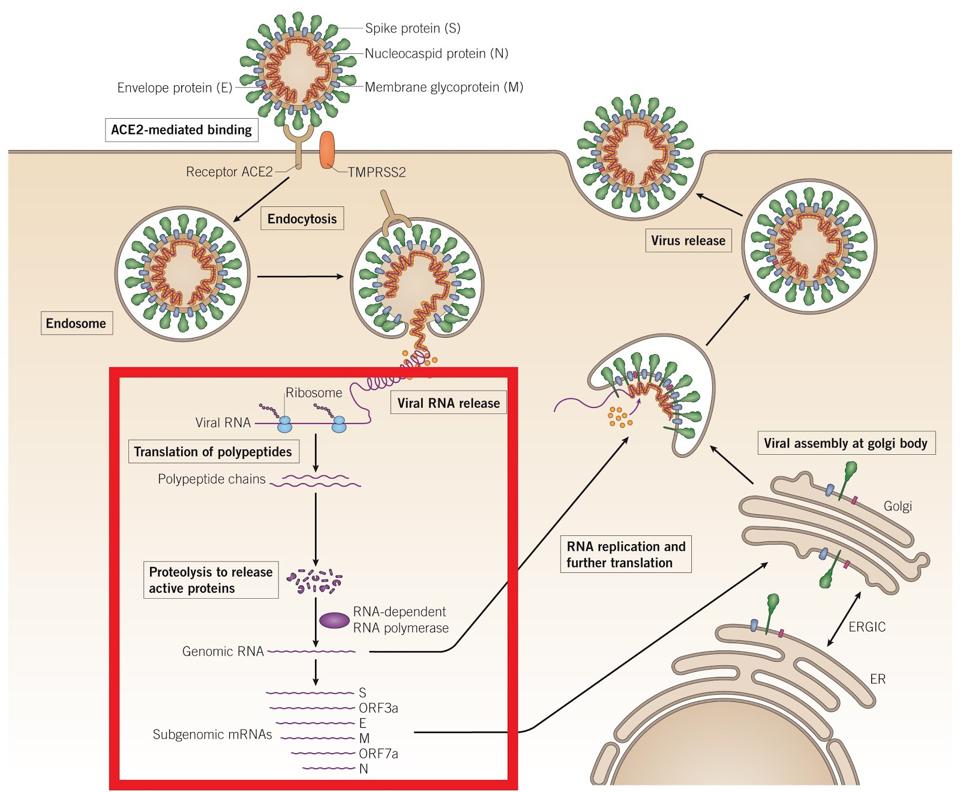
Early studies on Molnupiravir on SARS-CoV-2 in vitro indicated a logarithmic drop in virus production dependent on the dose of Molnupiravir introduced. This was possible because the RdRp of SARS-CoV-2 and SARS-CoV, which the drug was initially intended to treat, have 99.1% nucleotide similarity. Whole-genome deep-sequencing showed dose-dependent accumulation of random low-frequency mutations. Repeat exposure of virus populations to the drug was rapidly sterilizing, confirming that none of the random mutations mediate resistance to the drug. In the figures below, titer levels and genome production of SARS-CoV-2 decrease as the drug is introduced in higher volumes. At the time of the early study, mouse models and human testing were not yet available for Molnupiravir, but human trials began in June 2020.
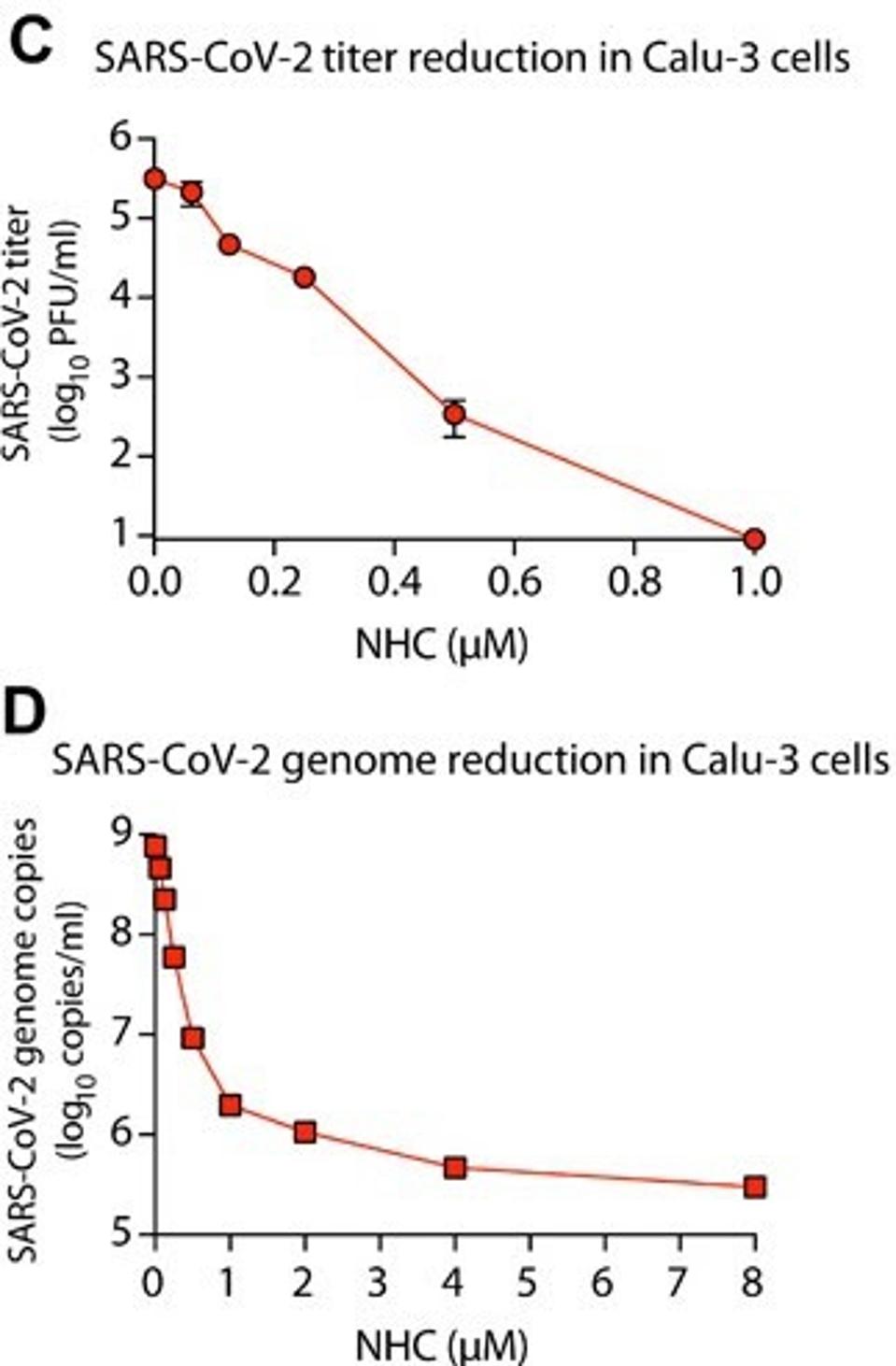
Reduction in SARS-CoV-2 titers after use of Molnupiravir
SHEAHAN ET AL. // UNC CHAPEL HILL
As detailed in a press release by Merck & Co, the preliminary results of these trials are promising. The trial was comprised of non-hospitalized adults who had symptoms of Covid-19 within seven days and confirmed SARS-CoV-2 infection.
The released details indicate that Molnupiravir in its full dosage prompts a reduction in days to negativity for the virus in nasopharyngeal swabs taken from participants with symptomatic SARS-CoV-2 infections. Five days after dose administration, 0% of those who received the dose were positive for the virus (0/47) compared with 24% of the placebo group (6/25). While details and study size is limited, a measurable drop in infection length is significant, as shorter infections may yield a smaller chance of transmission or severe symptoms.
The Merck press release follows a detailed study of this polymerase inhibitor in ferrets, given that ferrets and related members of the weasel genus can transmit viruses asymptomatically, resembling the human spread of viruses. Researchers found that this drug not only prevents close-quarter animal groupings from becoming as sick but the drug also reduces transmission of the virus. The figure below demonstrates the ferret study duration and test grouping. These results may very well be replicated in human trials when that data is released in the coming weeks.
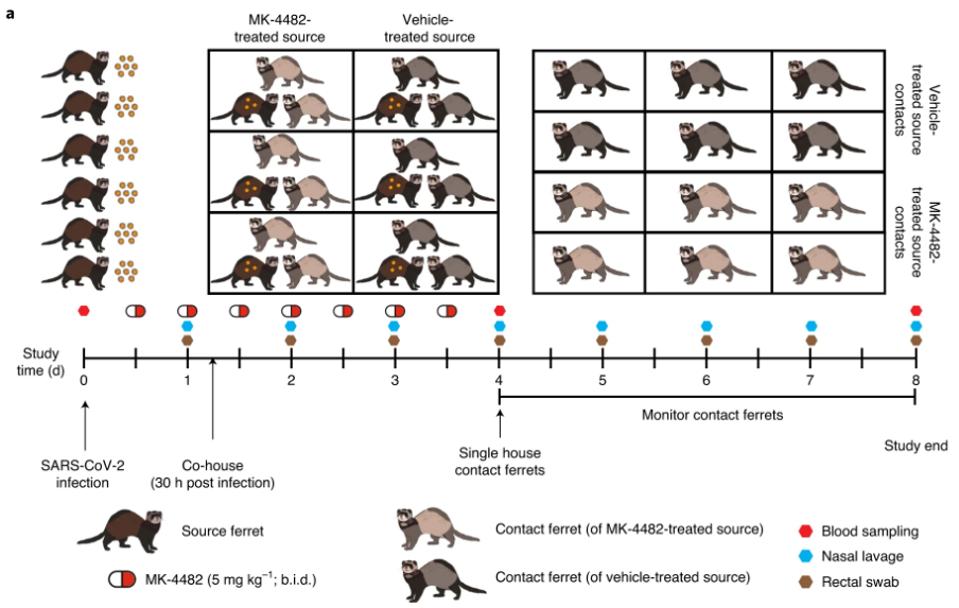
Ferret Molnupiravir treatment schedule
COX ET AL.
The positive results of Molnupiravir represent an emerging hope for more Covid-19 therapies to come. Its oral administration indicates a potential drug that could come before hospitalization and perhaps even prevent severe symptoms. Were a pill-based treatment for Covid-19 available, many lives would be easily saved and many hospital beds could be opened for those who need them.
In addition to its reduction of Covid-19 transmission, Molnupiravir is likely to be useful against influenza, ebola, and a large swath of other viruses as well. Its development appears to be a major advancement in virus control and should be active against Covid-19 variants and variants of other viruses. However, we caution Molnupiravir should be administered in conjunction with other therapies to avoid viruses rapidly developing resistance, which all these viruses are well-equipped to do.
Though, as these results are preliminary, we eagerly await the full release of the phase two data and the drug’s eventual full trial outcomes. This could be a real winner.
Read the full article on Forbes.
Originally published on March 16, 2021.