Novavax Covid-19 Vaccine Performs Well In Clinical Trials, But Variants Remain A Threat
(Posted on Tuesday, March 16, 2021)

SPAIN – 2021/03/02: In this photo illustration a hand holding a medical syringe in front of the Novavax logo. (Photo Illustration by Thiago Prudêncio/SOPA Images/LightRocket via Getty Images)
SOPA IMAGES/LIGHTROCKET VIA GETTY IMAGES
More than 330 million vaccinations have been administered in the fight against Covid-19, and a new vaccine candidate is now ready to enter the ring. Novavax recently completed its final analyses and will seek FDA and international approval in the coming weeks. The new candidate uses a mechanism to prompt immune responses that is different from vaccines already in circulation, but remains effective against non-variant forms of the virus. Here I discuss the results and implications of the Novavax trials and the vaccine’s performance against surging variants of SARS-CoV-2.
The press release details a United Kingdom trial of 15,000 participants. Against the wild-type strain of the virus, the two-dose vaccine posted 96.4% efficacy. Notably, the vaccine was also 100% effective in preventing severe disease, which included symptoms like tachypnea, high resting heart rate, required ventilation, hospitalization, among others.
Included in those 15,000 participants were also infections via the highly transmissible UK variant of the virus. The vaccine still posted an impressive 86.3% efficacy against the variant, which comes to an overall vaccine efficacy of 89.7% among the 15,000 participant study. Additionally, of non-placebo participants in the trial, only one case of Covid-19 was reported among those 65 and older, a demographic heavily hit by the pandemic.
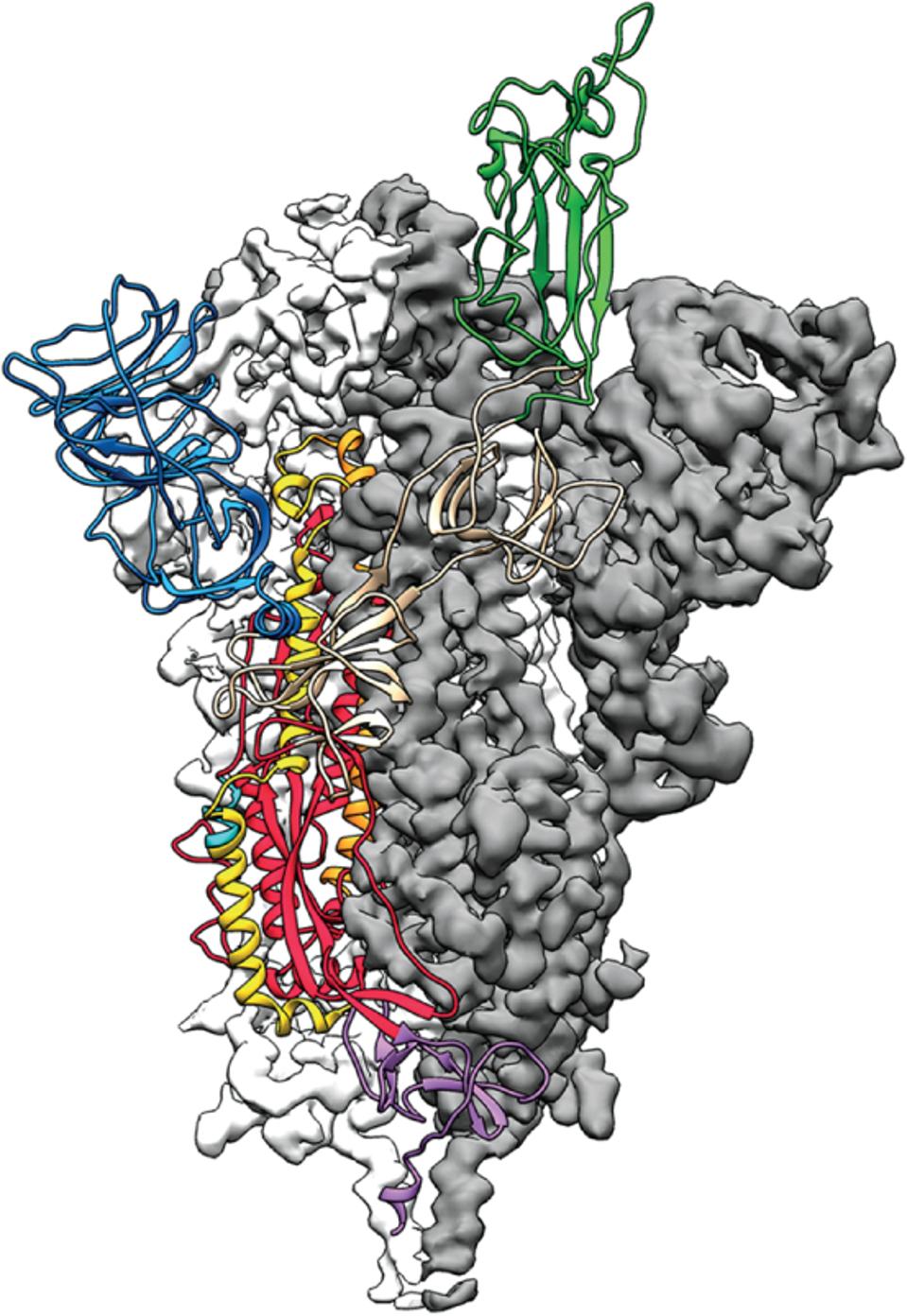
Structure of the nCoV trimeric spike.
“STRUCTURE OF THE NCOV TRIMERIC SPIKE” HTTPS://SCIENCE.SCIENCEMAG.ORG/CONTENT/367/6483/1207.3Novavax also wanted to see how its vaccine would fare against the antibody-resistant South African variant of the virus. Among a participant group of 2,905 adults, the vaccine demonstrated an overall efficacy of only 48.6% against the predominant B.1.351 variant. While no severe Covid-19 was noted in the vaccine group, these numbers are less encouraging than the UK group. Among HIV-negative adults, the efficacy rises to 55.4%, which is still well below par. This is a consistent theme across the vaccine landscape. The South African variant, along with its genetically similar counterparts like those in Brazil, Japan, and elsewhere are significantly resistant to vaccine-induced and naturally formed antibodies.
The Novavax vaccine works by combining a purified spike protein with an adjuvant, or a substance designed to enhance a given immune response. In 1984, I used essentially the same formula — adjuvant, envelope, and the equivalent of a spike — to create a vaccine that prevents cats from contracting leukemia. It is an old technology, but arguably the most direct line of attack we can mount against a virus. Other FDA-approved Covid-19 vaccines employ methods that are certainly innovative and still effective, but on the whole more roundabout approaches to hitting the same target. These include the messenger RNA in the Moderna and Pfizer-BioNTech vaccines, which instruct human cells to make spike proteins, and the adenovirus vectors in the Johnson & Johnson and AstraZeneca vaccines, which carry instructions for creating anti-SARS-CoV-2 antibodies.
That the clinical trials for the Novavax vaccine have yielded such promising results is a great sign, especially given the rise of new variants. Many pharmaceutical companies have already announced that they are developing second-generation vaccines tailored directly for these new variants. The only hesitations to note are which ones are they tailoring towards, and what if new ones arise in the time it takes to develop, test, and distribute? The illustration below shows some of the many variants that have branched off from the wild-type of SARS-CoV-2.
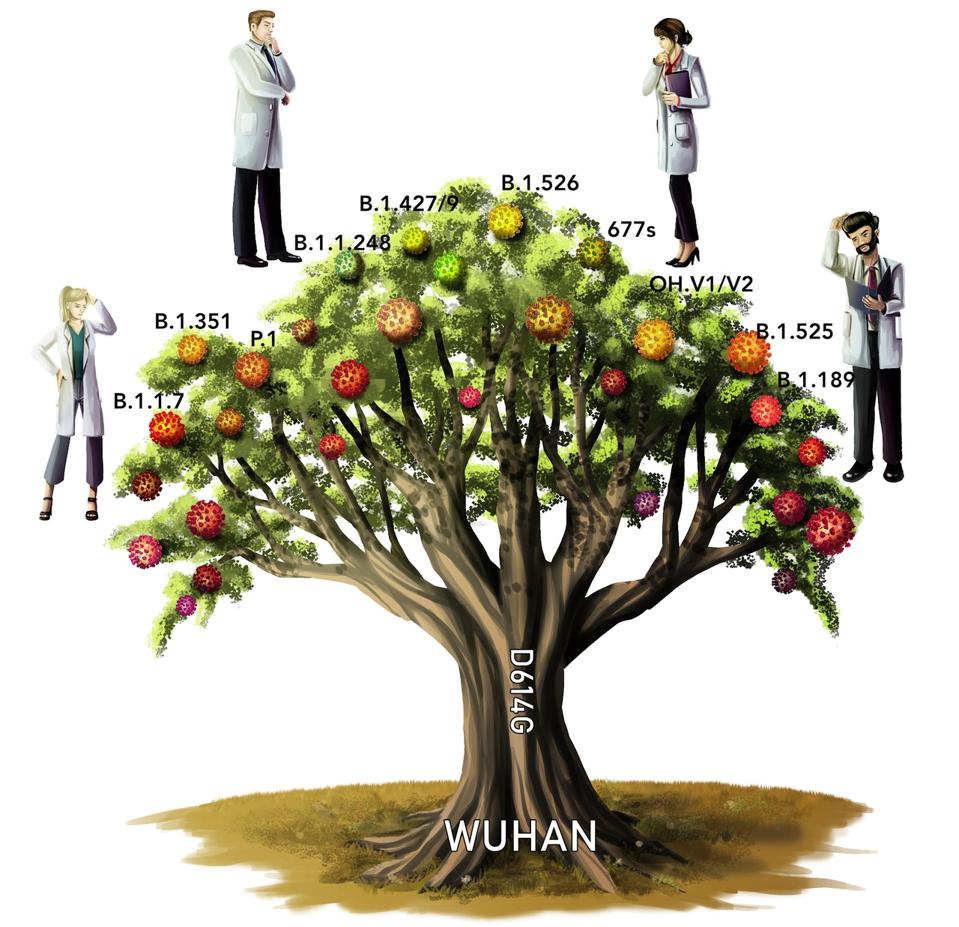
Figure 2. An illustration of a “variant tree.” The original Wuhan strain is the roots, the D614G mutation the trunk, and the new variants the fruits.
AUTHORRegardless, these hesitations should not take away from the positive news regarding this story. A new vaccine will soon be available that posts high efficacy towards the wild-type and one of the most prolific variants. Here’s hoping this vaccine will help reign in case numbers as new vaccines are developed in the coming months.
Originally published on Forbes (March 16, 2021)