Prophylactic Antibodies Alter Vaccine Responses To Covid-19
(Posted on Monday, March 6, 2023)
We are now in the third year of the Covid-19 pandemic. It is likely that Covid and the virus that causes it will be with us for many years. Most of us already have a complex history with Covid-19, including infection by the virus and exposure to a mix of vaccines and antiviral drugs. It is time to examine how the interplay of infections, antiviral drugs, and vaccines condition our response to new infections. Such a study is critical, as we now know that long-term protection from disease depends on the efficacy of memory response, not on initial neutralizing responses.
A recent study by Schaefer-Babajew et al. in the journal Nature begins to do just that by examining the influence of prophylactic antibody treatment on our antibody and memory B cells’ response to vaccines. Surprisingly, they find that protective antibody treatments significantly affect our B cell memory response and may diminish the ability of vaccines to protect us from serious diseases.
Study Design
Schaefer-Babajew et al. examined a cohort of 18 patients who received a combination antibody treatment of C144-LS and C135-LS, a combination yet to be approved or authorized for public use in the United States. Both of these antibodies bind to different regions of the receptor-binding domain and together have significant neutralizing potency against authentic SARS-CoV-2 wild-type virus obtained from human patients.
These antibodies were first described by Robbiani et al. in June 2020. C144 and C135 were isolated along with 50 other neutralizing antibodies from the sera of over 150 patients infected with SARS-CoV-2 in the early months of the pandemic. C144 and C135 were later modified with the ‘LS mutations,’ which is a method of improving the half-life of an antibody by enhancing the binding affinity between the Fc of an antibody and the human Fc receptor.
ROBBIANI ET AL.
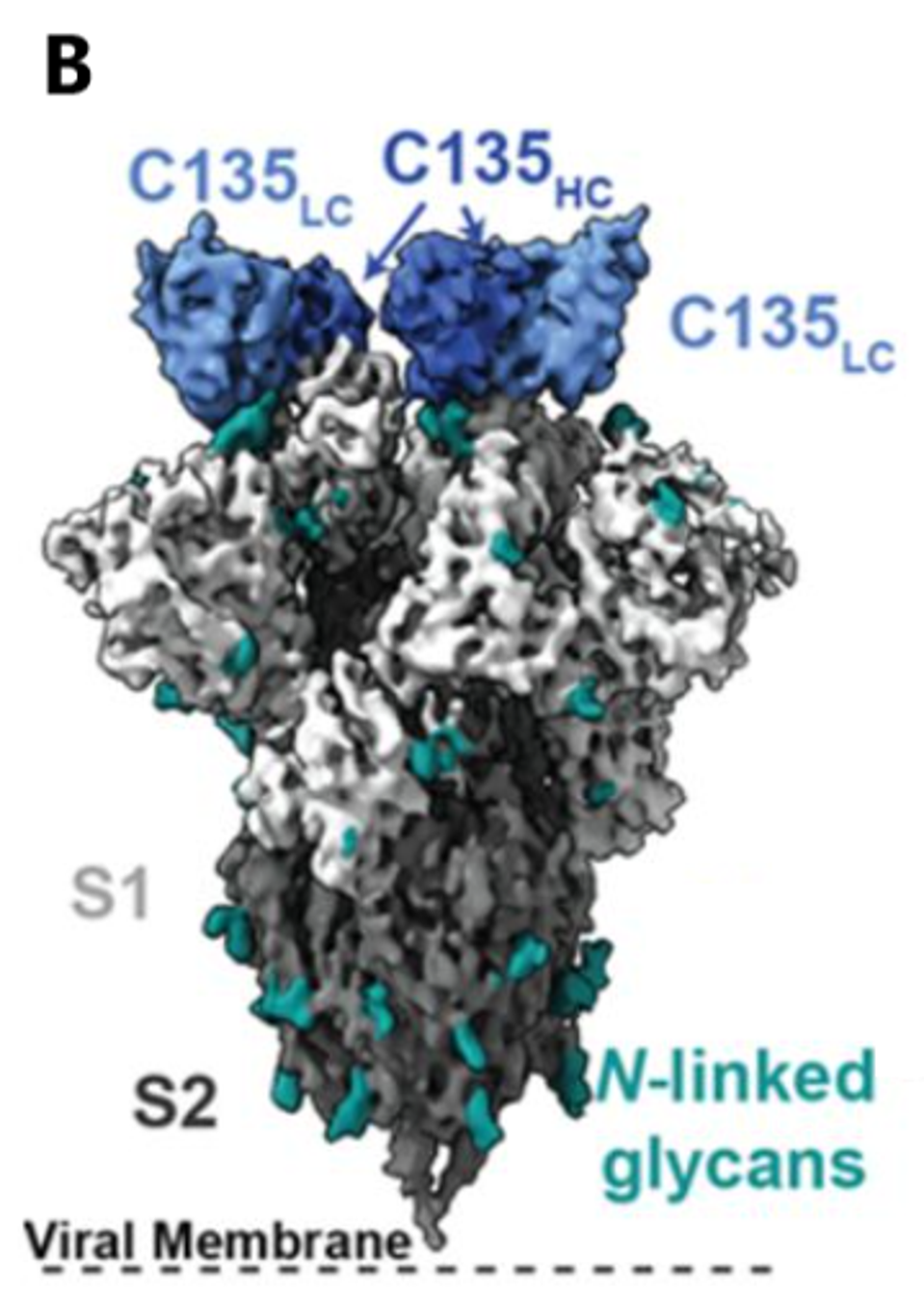
FIGURE 1: C144 (A) and C135 (B) binding epitope to SARS-CoV-2 spike. Cryo-EM density for the (A) C144–S trimer complex revealing C144 binding to a closed (three down RBDs) spike conformation and (B) C135–S trimer complex revealing C135 binding to an
ROBBIANI ET AL.
The cohort examined by Schaefer-Babajew et al. participated in a preliminary study to test the efficacy of these antibodies for submission to the Food and Drug Administration for emergency use authorization or approval. However, neither antibody is approved or authorized today. No patients in this study, whether experimental or control, were infected by SARS-CoV-2 before this study.
The 18 patients then received a two-dose mRNA vaccine regimen after a median of 82 days for the first dose and 103 days for the second. The cohort was compared to another group of patients vaccinated with two mRNA doses but with no previous history of infection or monoclonal antibody treatment. Patients received either the Moderna or Pfizer-BioNTech mRNA vaccine. The researchers examined the antibody binding, antibody neutralization, memory B cells, and antibodies produced by memory B cells.
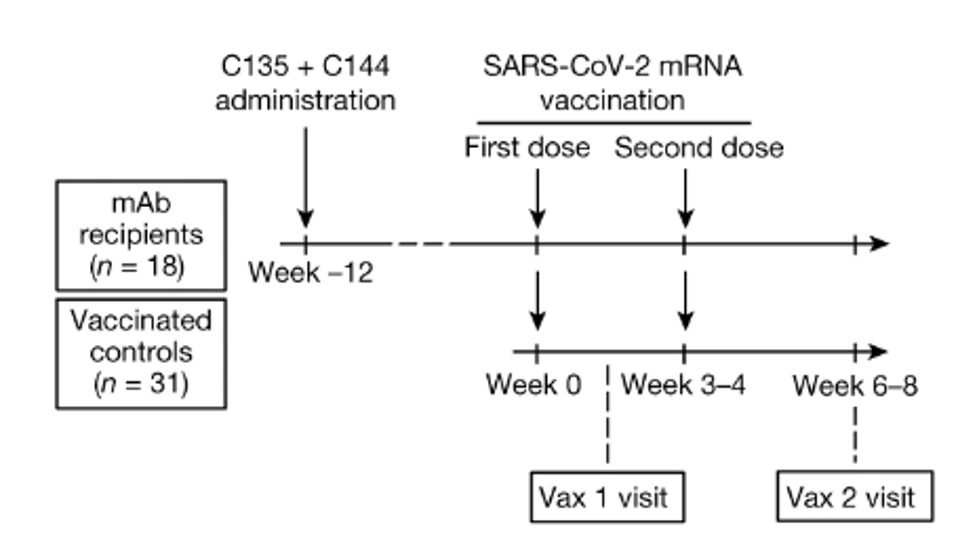
FIGURE 2: Schematic of the study design, with markers denoting weeks relative to the time of the first vaccine dose.
SCHAEFER-BABAJEW ET AL.
The Effect of Prior Treatment on Antibodies that Bind the Spike Protein
The first query was whether previous antibody treatment impacted antibody binding in patient sera. They examined the binding levels of two antibody types: IgM, which arises earlier in infection, and IgG, which is the most common type of antibody and is typically the type used for antibody treatments.
The IgM antibody binding efficiency between treatment and non-treatment patients was relatively stable. For IgG antibodies, binding in the treatment group rose above the non-treatment after the first dose, but stabilized after the second. These results, however, were solely when tested against the wild-type Wuhan virus.
When tested against a virus coded with receptor-binding domain mutations R346S/E484K or N440K/E484K, directly interfering with the epitope for both C144 and C135 binding, efficiency drops below the non-treatment group, though only marginally. The pseudovirus was also encoded with mutation R683G, far from the epitope involved with C144 and C135. The mutation disrupts the function of the furin-cleavage site, increasing particle infectivity without compromising the binding affinity between the receptor-binding domain and the antibodies.
Ultimately, the results show that monoclonal antibody infusions have little to no impact on IgM and IgG binding responses.
FIGURE 3: SARS-CoV-2 spike protein with notable mutations used in this study noted.
JASON MCLELLAN/UNIVERSITY OF TEXAS AT AUSTIN
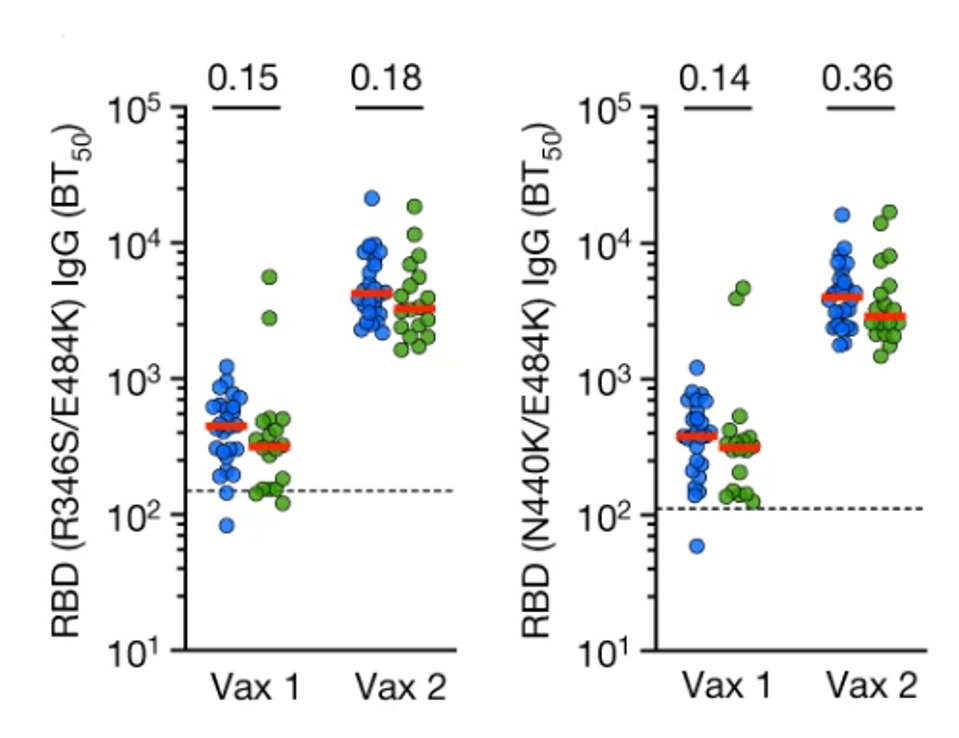
FIGURE 4: IgG binding to R346S/E484K (left) and N440K/E484K RBDs. Vaccinated controls are in blue, and mAb recipients are in green.
SCHAEFER-BABAJEW ET AL.
The Effect of Prior Treatment on Virus Neutralization
They next examined the effect of infused antibody treatment on post-vaccine neutralization. They again introduced C144 and C135 to a wild-type spike protein pseudovirus and a mutated version.
Against the wild-type, those who received the antibodies beforehand had significantly higher neutralizing titers after both the first and second doses. Their past monoclonal treatment enhanced their post-vaccine defense against the virus.
For the following mutated pseudoviruses, the antibodies made a negative impact rather than a positive one. The researchers introduced patient sera to pseudoviruses with R346S/Q493K and R346S/N440K/E484K mutations. Once again, R346S, N440K, Q493K, and E484K directly interfere with the binding epitopes of C144 and C135. After the first dose, those in the antibody group saw neutralizing titers fall 2.7-fold and 3.5-fold against the mutant viruses compared to the control group.
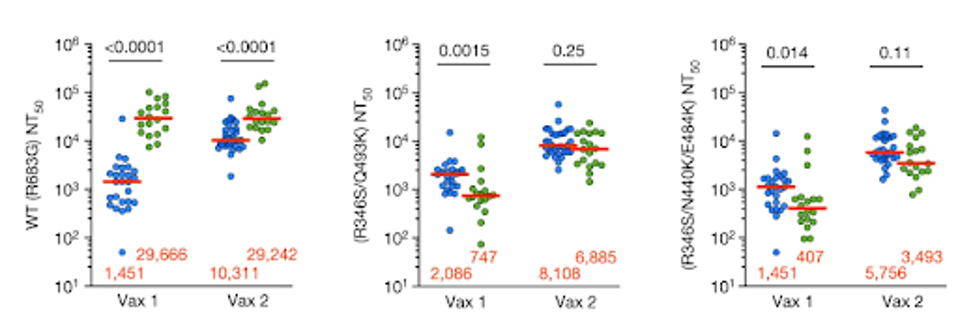
FIGURE 5: Plasma half-maximal neutralizing titer (NT50) values for monoclonal antibody recipients (n = 18, green) and controls (n = 26, blue) against HIV-1 pseudotyped with SARS-CoV-2 WT S (left), R346S/Q493K mutant S (middle) and R346S/N440K/E484K m
SCHAEFER-BABAJEW ET AL.
Neutralization rebounds to just a 0.6-fold to 0.85-fold drop from the control group after the second vaccination, which is less statistically significant, though still relevant. Against highly mutated spike proteins, previous antibody treatments may lower your immune defenses post-vaccination. This is notable as all viruses circulating today are heavily mutated in the receptor-binding domain, potentially causing concern for those with many previous antibody treatments.
The Effect of Prior Treatment on Memory B Cells
Perhaps the researchers’ most crucial finding relates to memory B cell responses. One critical aspect of memory is the persistence of memory B cells. These cells have full antibody maturation and are stable for months or even years. Reinfection by a similar virus causes rapid proliferation and production of protective B cell antibodies.
Schaefer-Babajew et al. found that mRNA vaccination elicited memory B cell responses approximately three-fold higher in the antibody group than in the control.
The Effect of Prior Treatment on Type and Neutralization of Antibodies Produced by Memory B Cells
Notably, the composition of the memory B cells was heavily altered in the antibody group. While the absolute number of IgG antibodies rose in the antibody group compared to the control, the relative percentage fell from a vast majority to just 45%. After the second dose, IgM antibodies catapulted from marginal levels in the control to 49% in the antibody group. The researchers attribute this to the pre-exposure to anti-RBD antibodies. IgM antibodies carry far fewer mutations than IgG, meaning the antibody treatment designed to neutralize the wild-type virus was potentially predisposed to favor IgM memory B cell expression.
Upon taking a closer look at the isolated antibodies from the antibody treatment group versus the control, some major concerns are revealed. To reiterate, IgM antibodies are much more heavily concentrated in the test group than in the control group.
First, examining the binding capacity of isolated antibodies, 62% of isolated antibodies bound the wild-type receptor binding domain poorly, if at all, compared to just 5% in the control group.
The results are worse when it comes to neutralization, as only 1 of the 45 IgG antibodies and none of the 32 IgM antibodies from the test group were neutralizing, as compared to 63% of the control IgG antibodies and 17% of the IgM antibodies.
These results are attributed to shifting the target epitope in the antibody group isolated memory B cell antibodies. Whereas half of the antibodies in the control group target epitope classes one, two, or three, just 20% do so in the treatment group, favoring the class four epitope.
Discussion
This study shows that preexisting treatment with anti-SARS-CoV-2 monoclonal antibodies significantly impacts the development of memory B cell responses in post-vaccinated patients. While the initial antibody levels were not harshly impacted, in some cases even increasing, memory B cell development suffered. Affinity thresholds for memory B cell development were lowered, leading to weaker antibodies that bound and neutralized relatively poorly compared to the control.
The increase in IgM memory also aligns with previous observations of rising IgM levels post-third and fourth doses of the mRNA vaccine. The shifting memory was accelerated in the patients that previously received monoclonal treatment. However, the increasing breadth of the memory antibody responses is countered by lacking neutralization and affinity.
This is not to say stay away from monoclonal antibody treatments. They can be life-saving in many instances and should be pursued early in infection, especially for those at high risk of severe disease progression. However, once you receive antibodies, your memory responses will be altered moving forward, and you may be at risk. Continue receiving mRNA boosters every three to six months to maximize immediate protection and lasting memory against SARS-CoV-2.
These studies are the first of what we hope will be many in examining the complex interaction between infection and both prophylactic and therapeutic interventions for Covid-19. The results show significant effects, particularly on memory B cells and the antibodies they produce. The consequence of these perturbations regarding protection from new variants remains to be seen, as new variants will inevitably arise. Nonetheless, we hope that these are the first of many studies investigating what is now a critical question three years into the pandemic.