To Guarantee Safety Of Covid-19 Vaccines, Prioritize Long-Term Studies
(Posted on Friday, November 20, 2020)

A medical syringe and vials in front of the COVID-19 word are seen in this creative photo taken on 18 November 2020. The U.S. biotechnology company Moderna announced its experimental Covid-19 vaccine with 94.5% effectiveness, as the media reported on
NURPHOTO VIA GETTY IMAGES
In the past few weeks, Covid-19 vaccines created by Pfizer and Moderna have made headlines with their promising phase 3 trial results. Neck-in-neck in efficacy, both reported reductions in Covid-19 related disease higher than 90 percent. The Food and Drug Administration (FDA) could open the doors to widespread use of these vaccines, either through expanded access or emergency use authorization, as early as next month.
Although the reviews leading up to a stamp of regulatory approval are proceeding full speed ahead, one last bend in the road awaits—phase 4 (see Figure 1). This stage is designed to catch any adverse effects that didn’t surface or register as significant over the course of clinical trials. It also involves monitoring the production of the vaccines themselves. Given the accelerated timeline, long-term followup will be of particular importance for any Covid-19 vaccine authorized for human use under any authority, and a new commentary in Science Magazine shows why.
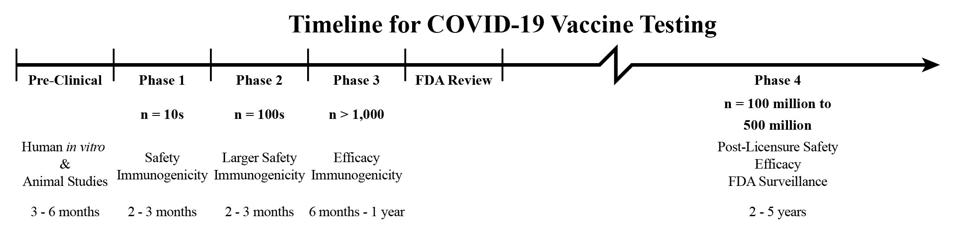
Figure 1: A timeline of safety evaluation for vaccines, adjusted to reflect the current pace of clinical trials and the necessary duration of post-licensure surveillance. Adapted from the original:
AUTHOR
While vaccines may be one of the most successful scientific technologies ever invented, the authors acknowledge, throughout history several have failed us. Many of the vaccine regulations we have today were borne of lessons learned the hard way—a contaminated batch that went on to infect thousands, or a rare side effect far more prevalent than initially perceived. A vaccine that fails to serve its original purpose, the safe prevention of disease, doesn’t just damage human health. It also triggers a loss of public confidence and fuels the rejection of future vaccines. But these fatal mistakes, contrary to what proponents of the anti-vax movement believe, are entirely avoidable, so long as we take care not to repeat them.
Sometimes the issue isn’t necessarily the vaccine technology itself, but a fundamental lack of quality control. Take, for instance, the so-called Cutter Incident of 1955. Cutter Laboratories was one of the pharmaceutical companies licensed to manufacture the Salk polio vaccine, which successfully used inactivated virus to stimulate bodily defenses against a horrific disease. Two of their batches, however, were mistakenly loaded up with live virus—poised, in other words, to spread polio rather than stop it. Those two batches alone went on to infect about 40,000 people, paralyzing 51 and killing five.
Another problem that can arise once a vaccine reaches the general public is vaccine-induced enhancement. When clinical studies fail to correctly determine the correlates of protection against disease, a vaccine can end up enhancing infection, rather than preventing it. Such was the case with an inactivated vaccine for respiratory syncytial virus (RSV) developed in the 1960s, approved because it generated a moderate antibody response in volunteers. The majority of children who received the vaccine ended up hospitalized. It was later found that the antibodies stimulated by the inactivated RSV vaccine weren’t neutralizing, and in fact appeared to contribute to disease enhancement.
Last but not least is the question of adverse effects that don’t raise alarm in clinical trials, but go on to develop in a significant minority of people who receive the vaccine. For the first rotavirus vaccine, authorized in 1998, the side effect was intussusception, a rare but potentially fatal condition that involves obstruction of the intestines and all the food and fluids that flow through them. For an influenza vaccine tested and administered in 1976, it was Guillain-Barré syndrome, a deadly autoimmune disorder that targets and harms nerve cells. Five cases of intussusception were documented in the rotavirus vaccine phase 3 trial, followed by 15 more in its first year on the market—a prevalence high enough to warrant suspension. The 1976 influenza vaccine, determined to be safe after a 7,000-patient trial, was terminated after causing about 450 cases of Guillain-Barré syndrome in a quarter of the U.S. population.
The faulty rotavirus and influenza vaccines, like the Cutter Incident and 1960s RSV vaccine, show us how a single oversight or misstep in the development of a vaccine can have untold consequences for the people who ultimately receive it. Today, as part of phase 4, regulatory agencies are required to closely inspect designated manufacturing facilities, test fresh batches of vaccines for purity and strength, and track reports of severe side effects using the Vaccine Adverse Event Reporting System. The FDA can also choose to implement phase 4 clinical trials that last years after a vaccine is licensed, a more active and scientifically rigorous approach to long-term follow-up.
Another disaster not only can be prevented, but must be, especially if hundreds of millions are lining up for a dose of the first Covid-19 vaccine to hit the shelves. While the current FDA guidelines for Covid-19 vaccine authorizations allude to the continuation of clinical trials following approval, extra care must be taken to enforce and preserve the integrity and longevity of phase 4 safety and monitoring procedures. If we aren’t sufficiently cautious and methodical in our approach, we risk once again making history—and not the good kind.
Originally published on Forbes (November 20, 2020)