Why Don’t We Have A Coronavirus Drug Yet—And How We Can Develop One As Soon As Possible
(Posted on Friday, April 3, 2020)
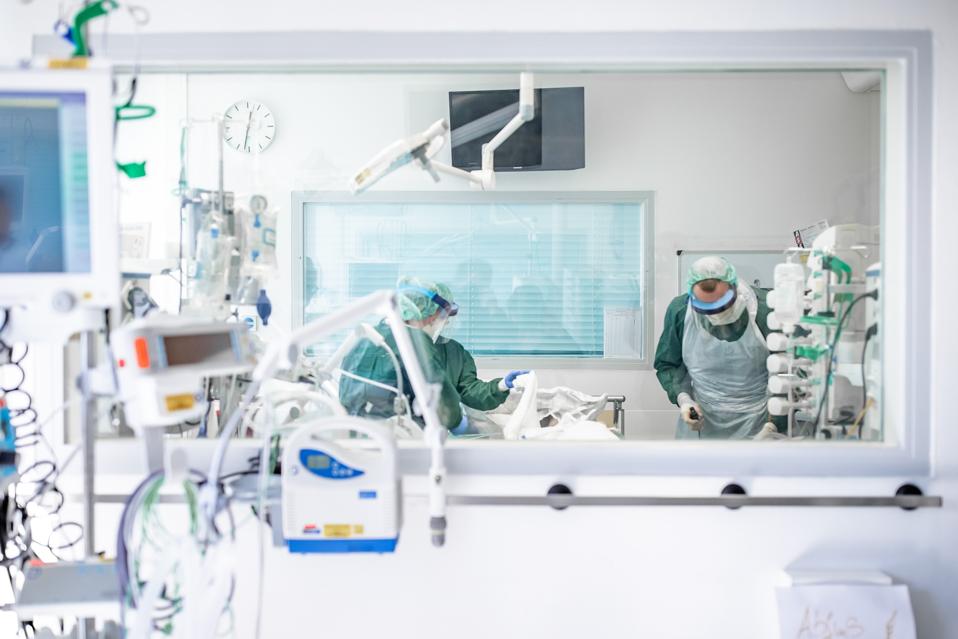
Nurses work in protective clothing in a hospital room in the intensive care unit of Essen University Hospital, where a corona patient from France is being treated. Photo: Marcel Kusch/dpa (Photo by Marcel Kusch/picture alliance via Getty Images)
DPA/PICTURE ALLIANCE VIA GETTY IMAGES
How will the new coronavirus pandemic end?
It could prove to be seasonal, meaning it peters out with the weather with a chance of returning at this time next year. A significant plurality of all people on Earth could contract the disease—prolonging its duration, slowing it over time through a gradual buildup of herd immunity, and inevitability leading to the death of millions. Or one of the many pharmaceutical companies hard at work on inventing a vaccine could succeed and administer their product widely and cheaply, though at least a year will go by before this comes to pass.
Our best option—the option that will save the most lives in the least amount of time—is to accelerate the development of therapeutic antiviral drugs that treat infection and prophylactic antiviral drugs that prevent infection. Two approaches can realistically achieve this. The first is to repurpose existing antivirals. The second is to develop de novo, from scratch. Pharmaceutical companies and national health agencies have begun to pursue both strategies aggressively as the Covid-19 outbreak intensifies.
Lucky for them, a massive corpus of laboratory studies conducted around past coronavirus outbreaks already exists—remnants of drug discovery efforts marshaled around SARS (Severe Acute Respiratory Syndrome) and MERS (Middle East Respiratory Syndrome) that never came to fruition. Much of the preclinical and phase I clinical trials showed promise and, had they advanced to the stages required for FDA approval, we might have had therapeutic or even prophylactic antivirals in our possession on the eve of Covid-19. What happened to halt the pipeline then, and how can we accelerate it now?
SARS & MERS: Why a drug was never developed
The origins of the SARS outbreak can be traced back to November 2002, when cases of an “atypical pneumonia” first appeared in the Guangdong Province of southern China. Come February 2003, similar reports were surfacing in regions as near as Hong Kong and countries distant as Canada. These outbreaks, previously thought to be isolated, began to occur with more frequency in March, mainly in hospital settings and therefore mainly infecting healthcare workers. By early April, a slew of research groups had determined a novel coronavirus to be the epidemic’s likeliest causative agent.
Initially, the international research response was robust. Once the virus was identified, diagnostic assays and profiles of its clinical, virological, and epidemiological characteristics soon followed. Potential antiviral candidates were investigated, animal models were developed—mice, macaque monkeys, and Golden Syrian hamsters among them—and vaccine strategies were charted and advanced, sometimes as far as phase I clinical trials. In the meantime, most patients infected with the disease were treated using experimental combinations of ribavirin, interferons, steroids, and antibiotics, though it was never proven at the time whether these therapeutic interventions corresponded with actual rates of recovery.
In July 2003, around 8,000 cases and 800 fatalities later, SARS was deemed to be officially contained. Efforts to research the SARS coronavirus, of which much remained unknown, continued, motivated by the certainty that it wouldn’t be the first to wreak havoc on human life. The lack of knowledge around the molecular biology of SARS-CoV, i.e. ideal targets for entry and replication inhibitors, that impeded drug development during the outbreak was largely resolved in its wake. All the pieces needed to bring new antivirals to the finish line were falling into place.
All except for one: the money. The funding streams funneled by pharmaceutical companies, governments, and nongovernmental organizations into labs around the world had all but dried up by 2006. No cases of SARS had been reported since 2004, and previously invested parties were losing interest. Thousands of scientific papers had been published on SARS, and yet nearly a decade later, when MERS first appeared in Saudi Arabia in late 2012, not a single antiviral drug was available for public consumption.
Since some of the more promising inhibitors identified in the SARS literature had yet to undergo large scale testing for toxicity, healthcare workers treating MERS stricken patients up and down the Arabian Peninsula reverted back to the experimental therapies administered to SARS patients—even though they were ultimately found to yield “little to no clinical benefit.” The MERS epidemic went on to infect fewer people than its predecessor—around 2,500, to be exact—but racked up a mortality rate triple that of SARS.
At first, it seemed like the momentum for finding antivirals had mounted anew. Calls came more urgently not just for a MERS vaccine, but a broad spectrum antiviral that could successfully neutralize future coronavirus threats. Yet by 2016, the year MERS cases definitively began to dwindle, the antiviral drug to make it furthest down the pipeline was only just entering phase I dose escalation trials. Three years later, that number had increased to three. The estimated date of completion? Ten years from now.
Covid-19: Paying for our past mistakes and avoiding new ones

SARS Inhibited (2006) stands in the center of the science city Biopolis in Singapore. The bronze sculpture by Mara Haseltine depicts the three dimensional polypeptide backbone of the active site of the SARS protease. www.calamara.com/
MARA HASELTINE
As of April 3, Covid-19 has infected more than one million and killed nearly 54,000. The tally has increased so exponentially that it has become difficult to keep track. From the beginning, it has already been too late; we had almost two decades to prepare ourselves and instead met our enemy unarmed.
Back when SARS research was still ongoing, the Singapore based biomedical research hub Biopolis commissioned a sculpture for their central plaza. SARS Inhibited (2006), the winning design conceived and actualized by artist Mara G. Haseltine, was erected as an homage to the notable contributions that Biopolis scientists made to the coronavirus drug discovery and development front. While their findings were unduly shelved, they are now seeing the light of day.
The Covid-19 coronavirus is called SARS-CoV-2 for a reason. While their genomes aren’t exactly identical, the two coronaviruses have between 80 to 90 percent of the same genetic material. Like SARS-CoV, SARS-CoV-2 penetrates a human lung cell by binding to ACE2, a receptor protein located on its surface. In an effort to expedite the search for efficient therapies, scientists are revisiting most—if not all—of the molecular targets identified some fifteen years ago in preclinical studies for SARS antivirals. Three targets emerged as particularly viable then that remain so today: the helicase, the RNA-dependent RNA polymerase, and the protease, depicted in SARS Inhibited.
The protease of coronaviruses is crucial to its replication, breaking down viral proteins into the functional units the virus needs to speedily copy itself. A clinical trial was held to determine whether lopinavir-ritonavir, a combination HIV treatment that inhibits coronavirus proteases, would deter the virus in Covid-19 patients. Unfortunately, as the researchers recently reported in an article for the New England Journal of Medicine, the drug failed to cure or diminish infection. That said, the unimpressive results aren’t conclusive, and testing of lopinavir-ritonavir will continue under the auspices of the World Health Organization multinational SOLIDARITY trial set to take place in Iran, South Africa, Switzerland, and elsewhere.
The RNA-dependent RNA polymerase is the target of Remdesivir, a broad spectrum antiviral invented by Gilead Sciences ten years ago. Remdesivir is designed to keep the RNA-dependent RNA polymerase from making copies of the virus’s genetic material. The drug was unsuccessful in stopping this process in the cells of Ebola patients, but its known safety in humans helped fast track clinical trials testing its efficacy for Covid-19 to phase III. Despite this, its prospects remain hazy; a Gilead analyst described the pipeline as “risky” and the outlook as “unclear.” Along with lopinavir-ritonavir, Remdesivir awaits further vetting via the SOLIDARITY trial, while the first outcomes of the trials taking place in the United States and China are due for an April release. Several other drugs are being tested, though none specific to SARS-C0V-2. Current evidence suggests they will be weakly effective in preventing and treating the infection at best.
Like the protease and RNA-dependent RNA polymerase, the helicase makes an ideal target due to the multifaceted role it plays in viral replication. While the body of knowledge on effective helicase inhibitors has been passed over in favor of more developed therapies, they still merit further investigation—as do the many other proteins that allow the coronavirus to commandeer our cellular machinery, whether they originally belong to the virus or the host. Already hot on this trail are hundreds of scientists who joined forces to map the interactions that individual parts of SARS-CoV-2 have with individual parts of our lung cells.
If the coronavirus uses these components to replicate, navigate, or otherwise destroy our cells’ defenses, then a drug that interferes with those interactions—of which there are reportedly more than 300—might be effective in stopping infection altogether. On Sunday, March 22, several of the scientists published a preliminary report of their findings that identified 69 drugs that might perform this function. Of these, 32 are undergoing preclinical development, 12 are in clinical trials, and 25 are already FDA-approved.
For now, it is ultimately these FDA-approved drug candidates—both those targeting viral proteins and those targeting human proteins—that offer us the most rapid solution. However, for now we do not have enough evidence to confirm their effectiveness as a treatment for Covid-19. In my view, the best solution would be a combination of virus specific antiviral drugs that inhibit the mechanisms the virus needs to reproduce itself.
If one or more drugs, alone or in combination, are shown to be effective in treating infection, those same drugs should also be used to prevent infection in the first place. This practice has a well-established precedent in antimalaria drugs, which are prescribed to people traveling to or stationed in countries where malaria is prevalent, and the combination antiviral drugs for HIV known as PrEP, recommended for populations at risk. There is much reason to believe that antivirals that effectively treat coronavirus infection will also be effective in preventing the infection of those exposed.
The horizon for vaccine development is too distant to even speak of, and though investigations of plasma derived and antibody based therapies are in the mix, whether these can be produced and deployed en masse is, for now, highly uncertain. The efficacy of drugs like chloroquine, which treat the respiratory consequences of infection but don’t attack the virus directly, is also not yet known.
We cannot allow a broad spectrum antiviral drug that targets all coronaviruses, not just Covid-19, to lay waste to the same fate that befell SARS and MERS. The laws of nature are not dictated by market forces—any snapshot of the global economy from the last few weeks will say as much. If we wish to survive this pandemic and the next, we must leverage federal agencies like BARDA and emergency funding mechanisms like Project BioShield to accelerate the effort now and continue it even when Covid-19 is well behind us. Otherwise, it won’t truly be the end—it will be just the beginning of many pandemics to come.
Originally published on Forbes (April 3, 2020)